CFTR limits F-actin formation and promotes morphological alignment with flow in human lung microvascular endothelial cells
- PMID: 34851051
- PMCID: PMC8634629
- DOI: 10.14814/phy2.15128
CFTR limits F-actin formation and promotes morphological alignment with flow in human lung microvascular endothelial cells
Abstract
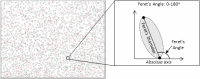
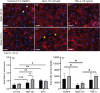
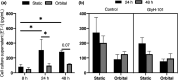
Micro- and macrovascular endothelial dysfunction in response to shear stress has been observed in cystic fibrosis (CF), and has been associated with inflammation and oxidative stress. We tested the hypothesis that the cystic fibrosis transmembrane conductance regulator (CFTR) regulates endothelial actin cytoskeleton dynamics and cellular alignment in response to flow. Human lung microvascular endothelial cells (HLMVEC) were cultured with either the CFTR inhibitor GlyH-101 (20 µM) or CFTRinh-172 (20 µM), tumor necrosis factor (TNF)-α (10 ng/ml) or a vehicle control (0.1% dimethyl sulfoxide) during 24 and 48 h of exposure to shear stress (11.1 dynes/cm2 ) or under static control conditions. Cellular morphology and filamentous actin (F-actin) were assessed using immunocytochemistry. [Nitrite] and endothelin-1 ([ET-1]) were determined in cell culture supernatant by ozone-based chemiluminescence and ELISA, respectively. Treatment of HLMVECs with both CFTR inhibitors prevented alignment of HLMVEC in the direction of flow after 24 and 48 h of shear stress, compared to vehicle control (both p < 0.05). Treatment with TNF-α significantly increased total F-actin after 24 h versus control (p < 0.05), an effect that was independent of shear stress. GlyH-101 significantly increased F-actin after 24 h of shear stress versus control (p < 0.05), with a significant (p < 0.05) reduction in cortical F-actin under both static and flow conditions. Shear stress decreased [ET-1] after 24 h (p < 0.05) and increased [nitrite] after 48 h (p < 0.05), but neither [nitrite] nor [ET-1] was affected by GlyH-101 (p > 0.05). CFTR appears to limit cytosolic actin polymerization, while maintaining a cortical rim actin distribution that is important for maintaining barrier integrity and promoting alignment with flow, without effects on endothelial nitrite or ET-1 production.
Keywords: actin cytoskeleton; cystic fibrosis; pulmonary microvascular endothelium; shear stress.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
There are no conflict of interest to report.
Figures



Similar articles
-
Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) in Human Lung Microvascular Endothelial Cells Controls Oxidative Stress, Reactive Oxygen-Mediated Cell Signaling and Inflammatory Responses.Front Physiol. 2020 Jul 29;11:879. doi: 10.3389/fphys.2020.00879. eCollection 2020. Front Physiol. 2020. PMID: 32848840 Free PMC article.
-
Revisiting CFTR inhibition: a comparative study of CFTRinh -172 and GlyH-101 inhibitors.Br J Pharmacol. 2014 Aug;171(15):3716-27. doi: 10.1111/bph.12726. Br J Pharmacol. 2014. PMID: 24758416 Free PMC article.
-
Mechanosensitive activation of CFTR by increased cell volume and hydrostatic pressure but not shear stress.Biochim Biophys Acta. 2015 Nov;1848(11 Pt A):2942-51. doi: 10.1016/j.bbamem.2015.09.009. Epub 2015 Sep 7. Biochim Biophys Acta. 2015. PMID: 26357939
-
Role of the actin cytoskeleton in the regulation of the cystic fibrosis transmembrane conductance regulator.Exp Physiol. 1996 May;81(3):505-14. doi: 10.1113/expphysiol.1996.sp003953. Exp Physiol. 1996. PMID: 8737083 Review.
-
Emerging relationship between CFTR, actin and tight junction organization in cystic fibrosis airway epithelium.Histol Histopathol. 2017 May;32(5):445-459. doi: 10.14670/HH-11-842. Epub 2016 Nov 11. Histol Histopathol. 2017. PMID: 27834058 Review.
Cited by
-
Elexacaftor-Tezacaftor-Ivacaftor improves exercise capacity in adolescents with cystic fibrosis.Pediatr Pulmonol. 2022 Nov;57(11):2652-2658. doi: 10.1002/ppul.26078. Epub 2022 Jul 22. Pediatr Pulmonol. 2022. PMID: 35851858 Free PMC article.
-
CFTR dictates monocyte adhesion by facilitating integrin clustering but not activation.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2412717122. doi: 10.1073/pnas.2412717122. Epub 2025 Jan 15. Proc Natl Acad Sci U S A. 2025. PMID: 39813254 Free PMC article.
-
CFTR as a therapeutic target for severe lung infection.Am J Physiol Lung Cell Mol Physiol. 2025 Feb 1;328(2):L229-L238. doi: 10.1152/ajplung.00289.2024. Epub 2025 Jan 8. Am J Physiol Lung Cell Mol Physiol. 2025. PMID: 39772994 Free PMC article. Review.
References
-
- Abbattiscianni, A. C. , Favia, M. , Mancini, M. T. , Cardone, R. A. , Guerra, L. , Monterisi, S. , Castellani, S. , Laselva, O. , Di Sole, F. , Conese, M. , Zaccolo, M. , & Casavola, V. (2016). Correctors of mutant CFTR enhance subcortical cAMP‐PKA signaling through modulating ezrin phosphorylation and cytoskeleton organization. Journal of Cell Science, 129, 1128–1140. - PubMed
-
- Bell, S. C. , Mall, M. A. , Gutierrez, H. , Macek, M. , Madge, S. , Davies, J. C. , Burgel, P. R. , Tullis, E. , Castaños, C. , Castellani, C. , Byrnes, C. A. , Cathcart, F. , Chotirmall, S. H. , Cosgriff, R. , Eichler, I. , Fajac, I. , Goss, C. H. , Drevinek, P. , Farrell, P. M. , … Ratjen, F. (2020). The future of cystic fibrosis care: A global perspective. Lancet Respiratory Medicine, 8, 65–124. - PMC - PubMed
-
- Blease, K. , Burke‐Gaffney, A. , & Hellewell, P. G. (1998). Modulation of cell adhesion molecule expression and function on human lung microvascular endothelial cells by inhibition of phosphodiesterases 3 and 4. British Journal of Pharmacology, 124, 229–237. 10.1038/sj.bjp.0701833 - DOI - PMC - PubMed
-
- Borcherding, D. C. , Siefert, M. E. , Lin, S. , Brewington, J. , Sadek, H. , Clancy, J. P. , Plafker, S. M. , & Ziady, A. G. (2019). Clinically approved CFTR modulators rescue Nrf2 dysfunction in cystic fibrosis airway epithelia. Journal of Clinical Investigation, 129, 3448–3463. 10.1172/JCI96273 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

