Herpes Simplex Virus 1 Small Noncoding RNAs 1 and 2 Activate the Herpesvirus Entry Mediator Promoter
- PMID: 34851143
- PMCID: PMC8826802
- DOI: 10.1128/JVI.01985-21
Herpes Simplex Virus 1 Small Noncoding RNAs 1 and 2 Activate the Herpesvirus Entry Mediator Promoter
Abstract
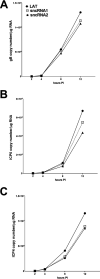
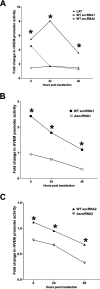
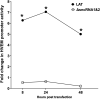
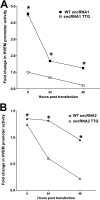
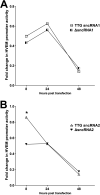
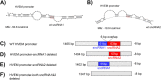
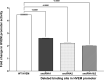
Herpes simplex virus 1 (HSV-1) latency-associated transcript (LAT) plays a significant role in efficient establishment of latency and reactivation. LAT has antiapoptotic activity and downregulates expression of components of the type I interferon pathway. LAT also specifically activates expression of the herpesvirus entry mediator (HVEM), one of seven known receptors used by HSV-1 for cell entry that is crucial for latency and reactivation. However, the mechanism by which LAT regulates HVEM expression is not known. LAT has two small noncoding RNAs (sncRNAs) that are not microRNAs (miRNAs), within its 1.5-kb stable transcript, which also have antiapoptotic activity. These sncRNAs may encode short peptides, but experimental evidence is lacking. Here, we demonstrate that these two sncRNAs control HVEM expression by activating its promoter. Both sncRNAs are required for wild-type (WT) levels of activation of HVEM, and sncRNA1 is more important in HVEM activation than sncRNA2. Disruption of a putative start codon in sncRNA1 and sncRNA2 sequences reduced HVEM promoter activity, suggesting that sncRNAs encode a protein. However, we did not detect peptide binding using two chromatin immunoprecipitation (ChIP) approaches, and a web-based algorithm predicts low probability that the putative peptides bind to DNA. In addition, computational modeling predicts that sncRNA molecules bind with high affinity to the HVEM promoter, and deletion of these binding sites to sncRNA1, sncRNA2, or both reduced HVEM promoter activity. Together, our data suggest that sncRNAs exert their function as RNA molecules, not as proteins, and we provide a model for the predicted binding affinities and binding sites of sncRNA1 and sncRNA2 in the HVEM promoter. IMPORTANCE HSV-1 causes recurrent ocular infections, which is the leading cause of corneal scarring and blindness. Corneal scarring is caused by the host immune response to repeated reactivation events. LAT functions by regulating latency and reactivation, in part by inhibiting apoptosis and activating HVEM expression. However, the mechanism used by LAT to control HVEM expression is unclear. Here, we demonstrate that two sncRNAs within the 1.5-kb LAT transcript activate HVEM expression by binding to two regions of its promoter. Interfering with these interactions may reduce latency and thereby eye disease associated with reactivation.
Keywords: HSV-1; HVEM; cornea; infection; luciferase; promoters; transfection; transfection systems; virus replication.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Small Noncoding RNA (sncRNA1) within the Latency-Associated Transcript Modulates Herpes Simplex Virus 1 Virulence and the Host Immune Response during Acute but Not Latent Infection.J Virol. 2022 Apr 13;96(7):e0005422. doi: 10.1128/jvi.00054-22. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254102 Free PMC article.
-
The anti-apoptotic function of HSV-1 LAT in neuronal cell cultures but not its function during reactivation correlates with expression of two small non-coding RNAs, sncRNA1&2.PLoS Pathog. 2024 Jun 10;20(6):e1012307. doi: 10.1371/journal.ppat.1012307. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38857310 Free PMC article.
-
Herpes Simplex Virus 1 Latency and the Kinetics of Reactivation Are Regulated by a Complex Network of Interactions between the Herpesvirus Entry Mediator, Its Ligands (gD, BTLA, LIGHT, and CD160), and the Latency-Associated Transcript.J Virol. 2018 Nov 27;92(24):e01451-18. doi: 10.1128/JVI.01451-18. Print 2018 Dec 15. J Virol. 2018. PMID: 30282707 Free PMC article.
-
Human alpha-herpesvirus 1 (HSV-1) viral replication and reactivation from latency are expedited by the glucocorticoid receptor.J Virol. 2025 Apr 15;99(4):e0030325. doi: 10.1128/jvi.00303-25. Epub 2025 Mar 27. J Virol. 2025. PMID: 40145740 Free PMC article. Review.
-
Herpes simplex virus type 1 and bovine herpesvirus 1 latency.Clin Microbiol Rev. 2003 Jan;16(1):79-95. doi: 10.1128/CMR.16.1.79-95.2003. Clin Microbiol Rev. 2003. PMID: 12525426 Free PMC article. Review.
Cited by
-
TRIM Proteins: Key Regulators of Immunity to Herpesvirus Infection.Viruses. 2024 Nov 6;16(11):1738. doi: 10.3390/v16111738. Viruses. 2024. PMID: 39599852 Free PMC article. Review.
-
A review of HSV pathogenesis, vaccine development, and advanced applications.Mol Biomed. 2024 Aug 29;5(1):35. doi: 10.1186/s43556-024-00199-7. Mol Biomed. 2024. PMID: 39207577 Free PMC article. Review.
-
Binding of herpesvirus entry mediator (HVEM) and HSV-1 gD affect reactivation but not latency levels.PLoS Pathog. 2023 Sep 22;19(9):e1011693. doi: 10.1371/journal.ppat.1011693. eCollection 2023 Sep. PLoS Pathog. 2023. PMID: 37738264 Free PMC article.
-
Studies of Infection and Experimental Reactivation by Recombinant VZV with Mutations in Virally-Encoded Small Non-Coding RNA.Viruses. 2022 May 10;14(5):1015. doi: 10.3390/v14051015. Viruses. 2022. PMID: 35632756 Free PMC article.
-
Small Noncoding RNA (sncRNA1) within the Latency-Associated Transcript Modulates Herpes Simplex Virus 1 Virulence and the Host Immune Response during Acute but Not Latent Infection.J Virol. 2022 Apr 13;96(7):e0005422. doi: 10.1128/jvi.00054-22. Epub 2022 Mar 7. J Virol. 2022. PMID: 35254102 Free PMC article.
References
-
- Mott KR, Bresee CJ, Allen SJ, BenMohamed L, Wechsler SL, Ghiasi H. 2009. Level of herpes simplex virus type 1 latency correlates with severity of corneal scarring and exhaustion of CD8+ T cells in trigeminal ganglia of latently infected mice. J Virol 83:2246–2254. 10.1128/JVI.02234-08. - DOI - PMC - PubMed
-
- Perng GC, Dunkel EC, Geary PA, Slanina SM, Ghiasi H, Kaiwar R, Nesburn AB, Wechsler SL. 1994. The latency-associated transcript gene of herpes simplex virus type 1 (HSV-1) is required for efficient in vivo spontaneous reactivation of HSV-1 from latency. J Virol 68:8045–8055. 10.1128/jvi.68.12.8045-8055.1994. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

