Dose Optimization of Combined Linezolid and Fosfomycin against Enterococcus by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model
- PMID: 34851157
- PMCID: PMC8635129
- DOI: 10.1128/Spectrum.00871-21
Dose Optimization of Combined Linezolid and Fosfomycin against Enterococcus by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model
Abstract
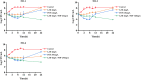
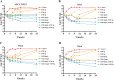
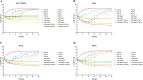
The rapid spread of antibiotic resistance among Enterococcus has prompted considerable interest in determining the dosage regimen of linezolid combined with fosfomycin. A checkerboard assay was employed to evaluate whether linezolid combined with fosfomycin had a synergistic effect on Enterococcus isolates from the hospital, including three drug-resistant strains (MIC of linezolid [MICLZD], ≥8 mg/L; MIC of fosfomycin [MICFOF], ≥256 mg/L). The in vitro static time-kill assay, dynamic pharmacokinetic (PK)/pharmacodynamic (PD) model, and semimechanistic PK/PD model were used to explore and predict effective combined dosage regimens. The checkerboard assay and in vitro static time-kill assay demonstrated that linezolid combined with fosfomycin has a synergistic effect on drug-resistant and sensitive Enterococcus. In the in vitro PK/PD model, the dosage regimen of linezolid (8 mg/L or 12 mg/L, steady-state concentration) combined with fosfomycin (6 g or 8 g) via a 0.5-h infusion every 8 h effectively suppressed bacterial growth at 24 h with a 3 log10 CFU/mL decrease compared with the initial inocula against two resistant and one sensitive Enterococcus isolates. The semimechanistic PK/PD model predicted that linezolid (more than 16 mg/L) combined with fosfomycin (6 g or 10 g) via a 0.5-h infusion every 8 h was required to achieve a 4 log10 CFU/mL decrease at 24 h against Enterococcus isolates (MICLZD ≥ 8 mg/L and MICFOF ≥ 256 mg/L). According to the prediction of the semimechanical PK/PD model, the effect of the combination was driven by linezolid, with fosfomycin enhancing the effect. Our study is the first to explore the synergistic effects of these two drugs from a qualitative and quantitative perspective and provides a simulation tool for future studies. IMPORTANCE In this study, we found that linezolid combined with fosfomycin could kill Enterococcus in vitro and that the administered dose was significantly lower after the combination treatment, which could reduce adverse effects and the development of drug resistance. The potential mechanism of the two-drug combination against Enterococcus was revealed from a quantitative perspective, which is an important step toward dose optimization in simulated humans. We hope that our research will help build a better relationship between clinicians and patients as we work together to address the challenges of antibiotic resistance in the 21st century.
Keywords: Enterococcus; PK/PD model; combination therapy; fosfomycin; linezolid.
Conflict of interest statement
We declare no conflicts of interest.
Figures



Similar articles
-
Dose Optimization of Colistin Combinations against Carbapenem-Resistant Acinetobacter baumannii from Patients with Hospital-Acquired Pneumonia in China by Using an In Vitro Pharmacokinetic/Pharmacodynamic Model.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e01989-18. doi: 10.1128/AAC.01989-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30745385 Free PMC article.
-
In vitro activities of daptomycin combined with fosfomycin or rifampin on planktonic and adherent linezolid-resistant isolates of Enterococcus faecalis.J Med Microbiol. 2019 Mar;68(3):493-502. doi: 10.1099/jmm.0.000945. J Med Microbiol. 2019. PMID: 30882300
-
Developments in the pharmacokinetic/pharmacodynamic index of linezolid: a step toward dose optimization using Monte Carlo simulation in critically ill patients.Int J Infect Dis. 2014 May;22:35-40. doi: 10.1016/j.ijid.2014.01.016. Epub 2014 Mar 4. Int J Infect Dis. 2014. PMID: 24603161
-
Treatment options for vancomycin-resistant enterococcal infections.Drugs. 2002;62(3):425-41. doi: 10.2165/00003495-200262030-00002. Drugs. 2002. PMID: 11827558 Review.
-
The revival of fosfomycin.Int J Infect Dis. 2011 Nov;15(11):e732-9. doi: 10.1016/j.ijid.2011.07.007. Epub 2011 Sep 25. Int J Infect Dis. 2011. PMID: 21945848 Review.
Cited by
-
Enhanced efficacy of sequential administration of fosfomycin and linezolid against methicillin-resistant Staphylococcus aureus.Front Microbiol. 2025 Mar 17;16:1511707. doi: 10.3389/fmicb.2025.1511707. eCollection 2025. Front Microbiol. 2025. PMID: 40165785 Free PMC article.
-
Antibiotic Resistance to Molecules Commonly Prescribed for the Treatment of Antibiotic-Resistant Gram-Positive Pathogens: What Is Relevant for the Clinician?Pathogens. 2024 Jan 19;13(1):88. doi: 10.3390/pathogens13010088. Pathogens. 2024. PMID: 38276161 Free PMC article. Review.
-
N6-methyltubercidin gives sterile cure in a cutaneous Leishmania amazonensis mouse model.Parasitology. 2024 Apr;151(5):506-513. doi: 10.1017/S0031182024000362. Epub 2024 Mar 27. Parasitology. 2024. PMID: 38533610 Free PMC article.
-
Oral Antibiotics for Bacteremia and Infective Endocarditis: Current Evidence and Future Perspectives.Microorganisms. 2023 Dec 18;11(12):3004. doi: 10.3390/microorganisms11123004. Microorganisms. 2023. PMID: 38138148 Free PMC article. Review.
-
Assessing the Emergence of Resistance in vitro and Invivo: Linezolid Combined with Fosfomycin Against Fosfomycin-Sensitive and Resistant Enterococcus.Infect Drug Resist. 2022 Aug 30;15:4995-5010. doi: 10.2147/IDR.S377848. eCollection 2022. Infect Drug Resist. 2022. PMID: 36065277 Free PMC article.
References
-
- Chirouze C, Athan E, Alla F, Chu VH, Ralph Corey G, Selton-Suty C, Erpelding ML, Miro JM, Olaison L, Hoen B, International Collaboration on Endocarditis Study Group. 2013. Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-Prospective Cohort Study. Clin Microbiol Infect 19:1140–1147. doi:10.1111/1469-0691.12166. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

