Age-Dependent Reduction in Neutralization against Alpha and Beta Variants of BNT162b2 SARS-CoV-2 Vaccine-Induced Immunity
- PMID: 34851162
- PMCID: PMC8635122
- DOI: 10.1128/Spectrum.00561-21
Age-Dependent Reduction in Neutralization against Alpha and Beta Variants of BNT162b2 SARS-CoV-2 Vaccine-Induced Immunity
Abstract
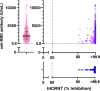
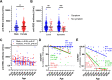
Vaccines against severe acute respiratory syndrome coronavirus-2 have been introduced. To investigate the relationship between vaccine-induced humoral immunity and patient age, we measured antibody levels and neutralization in vaccinated sera. Sera from 13 to 17 days after the second dose of the BNT162b2 vaccine were collected from health care workers at the University of Toyama (n = 740). Antibody levels were measured by the anti-receptor binding domain antibody test (anti-RBD test), and neutralization against wild-type (WT), α- and β-variant pseudotyped viruses were assayed using a high-throughput chemiluminescent reduction neutralizing test (htCRNT; positivity cutoff, 50% neutralization at serum dilution 1:100). Basic clinical characteristics were obtained from questionnaires. Antibodies were confirmed in all participants in both the anti-RBD test (median, 2,112 U/ml; interquartile range [IQR], 1,275 to 3,390 U/ml) and the htCRNT against WT (median % inhibition, >99.9; IQR, >99.9 to >99.9). For randomly selected sera (n = 61), 100.0% had positive htCRNT values against the α- and β-derived variants. Among those who answered the questionnaire (n = 237), the values of the anti-RBD test were negatively correlated with age in females (P < 0.01). An age-dependent decline in neutralization was observed against the variants but not against the wild-type virus (wild type, P = 0.09; α, P < 0.01; β, P < 0.01). The neutralizing activity induced by BNT162b2 was obtained not only against the wild-type virus, but also against the variants; however, there was an age-dependent decrease in the latter. Age-related heterogeneity of vaccine-acquired immunity is a concern in preventive strategies in the era dominated by variants. IMPORTANCE Since mRNA vaccines utilize wild-type SARS-CoV-2 spike protein as an antigen, there are potential concerns about acquiring immunity to variants of this virus. The neutralizing activity in BNT162b2-vaccinated individuals was higher against the wild-type virus than against its variants; this effect was more apparent in older age groups. This finding suggests that one of the weaknesses of the mRNA vaccine is the high risk of variant infection in the elderly population. Because the elderly are at a higher risk of SARS-CoV-2 infection, the age-dependent decline of neutralization against viral variants should be considered while planning vaccination programs that include boosters.
Keywords: BNT162b2; SARS-CoV-2; neutralizing antibodies; receptor-binding domain; variants.
Figures

Similar articles
-
Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants.Microbiol Spectr. 2021 Dec 22;9(3):e0056021. doi: 10.1128/Spectrum.00560-21. Epub 2021 Dec 1. Microbiol Spectr. 2021. PMID: 34851163 Free PMC article.
-
Diversified humoral immunity and impacts of booster vaccines: SARS-CoV-2 antibody profile and Omicron BA.2 neutralization before and after first or second boosters.Microbiol Spectr. 2024 Oct 3;12(10):e0060524. doi: 10.1128/spectrum.00605-24. Epub 2024 Aug 20. Microbiol Spectr. 2024. PMID: 39162540 Free PMC article.
-
Anti-SARS-CoV-2 Immunoglobulin Isotypes, and Neutralization Activity Against Viral Variants, According to BNT162b2-Vaccination and Infection History.Front Immunol. 2021 Dec 17;12:793191. doi: 10.3389/fimmu.2021.793191. eCollection 2021. Front Immunol. 2021. PMID: 34975897 Free PMC article.
-
Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis.Clin Infect Dis. 2022 Mar 1;74(4):734-742. doi: 10.1093/cid/ciab646. Clin Infect Dis. 2022. PMID: 34302458 Free PMC article.
-
Broadly-Neutralizing Antibodies Against Emerging SARS-CoV-2 Variants.Front Immunol. 2021 Sep 27;12:752003. doi: 10.3389/fimmu.2021.752003. eCollection 2021. Front Immunol. 2021. PMID: 34646276 Free PMC article. Review.
Cited by
-
Neutralization of Omicron subvariants BA.1 and BA.5 by a booster dose of COVID-19 mRNA vaccine in a Japanese nursing home cohort.Vaccine. 2023 Mar 24;41(13):2234-2242. doi: 10.1016/j.vaccine.2023.02.068. Epub 2023 Feb 27. Vaccine. 2023. PMID: 36858871 Free PMC article.
-
Evaluation of antibody responses in healthy individuals receiving SARS-CoV-2 inactivated vaccines.Biosaf Health. 2024 Apr 19;6(3):153-164. doi: 10.1016/j.bsheal.2024.04.001. eCollection 2024 Jun. Biosaf Health. 2024. PMID: 40078728 Free PMC article.
-
BNT162b2-induced neutralizing and non-neutralizing antibody functions against SARS-CoV-2 diminish with age.Cell Rep. 2022 Oct 25;41(4):111544. doi: 10.1016/j.celrep.2022.111544. Epub 2022 Oct 5. Cell Rep. 2022. PMID: 36252569 Free PMC article.
-
Antibody response to SARS-CoV-2 WT and Omicron BA.4/5 of inactivated COVID-19 vaccine in patients with lung cancer after second and booster immunization.J Hematol Oncol. 2023 May 3;16(1):47. doi: 10.1186/s13045-023-01443-3. J Hematol Oncol. 2023. PMID: 37138279 Free PMC article.
-
Inactivated SARS-CoV-2 booster vaccine enhanced immune responses in patients with chronic liver diseases.Virol Sin. 2023 Oct;38(5):723-734. doi: 10.1016/j.virs.2023.07.005. Epub 2023 Jul 22. Virol Sin. 2023. PMID: 37487943 Free PMC article.
References
-
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW, Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC, C4591001 Clinical Trial Group. 2020. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med 383:2603–2615. doi:10.1056/NEJMoa2034577. - DOI - PMC - PubMed
-
- Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, Holden KA, Read JM, Dondelinger F, Carson G, Merson L, Lee J, Plotkin D, Sigfrid L, Halpin S, Jackson C, Gamble C, Horby PW, Nguyen-Van-Tam JS, Ho A, Russell CD, Dunning J, Openshaw PJ, Baillie JK, Semple MG, ISARIC4C investigators. 2020. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ 369:m1985. doi:10.1136/bmj.m1985. - DOI - PMC - PubMed
-
- Müller L, Andrée M, Moskorz W, Drexler I, Walotka L, Grothmann R, Ptok J, Hillebrandt J, Ritchie A, Rabl D, Ostermann PN, Robitzsch R, Hauka S, Walker A, Menne C, Grutza R, Timm J, Adams O, Schaal H. 2021. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. Clin Infect Dis, In press. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

