Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants
- PMID: 34851163
- PMCID: PMC8635131
- DOI: 10.1128/Spectrum.00560-21
Correlation of the Commercial Anti-SARS-CoV-2 Receptor Binding Domain Antibody Test with the Chemiluminescent Reduction Neutralizing Test and Possible Detection of Antibodies to Emerging Variants
Abstract
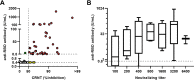
Serological tests are beneficial for recognizing the immune response against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). To identify protective immunity, optimization of the chemiluminescent reduction neutralizing test (CRNT) is critical. Whether commercial antibody tests have comparable accuracy is unknown. Serum samples were obtained from COVID-19 patients (n = 74), SARS-CoV-2 PCR-negative (n = 179), and suspected healthy individuals (n = 229) before SARS-CoV-2 variants had been detected locally. The convalescent phase was defined as the period after day 10 from disease onset or the episode of close contact. The CRNT using pseudotyped viruses displaying the wild-type (WT) spike protein and a commercial anti-receptor-binding domain (RBD) antibody test were assayed. Serology for the B.1.1.7 and B.1.351 variants was also assayed. Both tests concurred for symptomatic COVID-19 patients in the convalescent phase. They clearly differentiated between patients and suspected healthy individuals (sensitivity: 95.8% and 100%, respectively; specificity: 99.1% and 100%, respectively). Anti-RBD antibody test results correlated with neutralizing titers (r = 0.31, 95% confidence interval [CI] 0.22-0.38). Compared with the WT, lower CRNT values were observed for the variants. Of the samples with ≥100 U/mL by the anti-RBD antibody test, 77.8% and 88.9% showed ≥50% neutralization against the B.1.1.7 and the B.1.351 variants, respectively. Exceeding 100 U/mL in the anti-RBD antibody test was associated with neutralization of variants (P < 0.01). The CRNT and commercial anti-RBD antibody test effectively classified convalescent COVID-19 patients. Strong positive results with the anti-RBD antibody test can reflect neutralizing activity against emerging variants. IMPORTANCE This study provides a diagnostic evidence of test validity, which can lead to vaccine efficacy and proof of recovery after COVID-19. It is not easy to know neutralization against SARS-CoV-2 in the clinical laboratory because of technical and biohazard issues. The correlation of the quantitative anti-receptor-binding domain antibody test, which is widely available, with neutralizing test indicates that we can know indirectly the state of acquisition of functional immunity against wild and variant-type viruses in the clinical laboratory.
Keywords: convalescent; high throughput; neutralizing antibodies; receptor-binding domain; seroconversion.
Figures


References
-
- Tani H, Kimura M, Tan L, Yoshida Y, Ozawa T, Kishi H, Fukushi S, Saijo M, Sano K, Suzuki T, Kawasuji H, Ueno A, Miyajima Y, Fukui Y, Sakamaki I, Yamamoto Y, Morinaga Y. 2021. Evaluation of SARS-CoV-2 neutralizing antibodies using a vesicular stomatitis virus possessing SARS-CoV-2 spike protein. Virol J 18:16. doi:10.1186/s12985-021-01490-7. - DOI - PMC - PubMed
-
- Kawasuji H, Morinaga Y, Tani H, Kimura M, Yamada H, Yoshida Y, Takegoshi Y, Kaneda M, Murai Y, Kimoto K, Ueno A, Miyajima Y, Kawago K, Fukui Y, Sakamaki I, Yamamoto Y. 2021. Delayed neutralizing antibody response in the acute phase correlates with severe progression of COVID-19. Sci Rep 11:16535. doi:10.1038/s41598-021-96143-8. - DOI - PMC - PubMed
-
- Rubio-Acero R, Castelletti N, Fingerle V, Olbrich L, Bakuli A, Wölfel R, Girl P, Müller K, Jochum S, Strobl M. 2021. In search for the SARS-CoV-2 protection correlate: a head-to-head comparison of two quantitative S1 assays in a group of pre-characterized oligo-/asymptomatic patients. medRxiv. doi:10.1101/2021.02.19.21252080. - DOI - PubMed
-
- Bal A, Trabaud MA, Fassier JB, Rabilloud M, Saker K, Langlois-Jacques C, Guibert N, Paul A, Alfaiate D, Massardier Pilonchery A, Pitiot V, Morfin Sherpa F, Lina B, Pozzetto B, Trouillet Assant S, COVID SER Study Group. 2021. Six-month antibody response to SARS-CoV-2 in healthcare workers assessed by virus neutralization and commercial assays. Clin Microbiol Infect 27:933–935. doi:10.1016/j.cmi.2021.01.003. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

