Pseudomonas aeruginosa Mobbing-Like Behavior against Acanthamoeba castellanii Bacterivore and Its Rapid Control by Quorum Sensing and Environmental Cues
- PMID: 34851177
- PMCID: PMC8635135
- DOI: 10.1128/Spectrum.00642-21
Pseudomonas aeruginosa Mobbing-Like Behavior against Acanthamoeba castellanii Bacterivore and Its Rapid Control by Quorum Sensing and Environmental Cues
Abstract
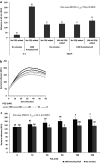
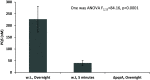
Mobbing, group attack of prey on predator, is a behavior seen in many animal species in which prey animals use numbers and coordination to counter individually superior predators. We studied attack behavior of Pseudomonas aeruginosa toward the bacterivore Acanthamoeba castellanii. This behavior consists of directed motility toward and specific adhesion to the predator cells, enacted in seconds and responding to both prey and predator population densities. Attack coordination relies on remote sensing of the predator and the use of the Pseudomonas quinolone signal (PQS), a P. aeruginosa species-specific quorum sensing molecule. Mutants unable to produce the PQS show unspecific adhesion and reduced survival, and a corresponding increase in predator population occurs as a result of predation. The addition of an external PQS restored some predator-specific adherence within seconds, suggesting a novel response mechanism to this quorum sensing (QS) signal. Fast behavioral response of P. aeruginosa to PQS is also supported by the rate of signal accumulation in the culture, reaching relevant concentrations within minutes, enabling bacteria response to self population density in these short timescales. These results portray a well-regulated group attack of the bacteria against their predator, reacting within seconds to environmental cues and species-specific signaling, which is analogous in many ways to animal mobbing behavior. IMPORTANCE Pseudomonas aeruginosa was shown previously to attack amoebae and other predators by adhering to them and injecting them with virulent substances. In this work, we show that an active, coordinated group behavior is enacted by the bacteria to utilize these molecular components, responding to both predator and bacterial population density. In addition to their ecological significance, immediate behavioral changes observed in response to PQS suggest the existence of a fast QS signal cascade, which is different from canonical QS that relies on slow-to-respond gene regulation. Similar regulatory circuits may drive other bacterial adaptations and pathogenicity mechanisms and may have important clinical implications.
Keywords: PQS; adhesion; biofilm; predation avoidance; quorum sensing.
Figures






Similar articles
-
Pseudomonas quinolone signalling system: a component of quorum sensing cascade is a crucial player in the acute urinary tract infection caused by Pseudomonas aeruginosa.Int J Med Microbiol. 2014 Nov;304(8):1199-208. doi: 10.1016/j.ijmm.2014.08.013. Epub 2014 Sep 1. Int J Med Microbiol. 2014. PMID: 25240873
-
Phage Infection Restores PQS Signaling and Enhances Growth of a Pseudomonas aeruginosa lasI Quorum-Sensing Mutant.J Bacteriol. 2022 May 17;204(5):e0055721. doi: 10.1128/jb.00557-21. Epub 2022 Apr 7. J Bacteriol. 2022. PMID: 35389255 Free PMC article.
-
Spatiotemporal Distribution of Pseudomonas aeruginosa Alkyl Quinolones under Metabolic and Competitive Stress.mSphere. 2020 Jul 22;5(4):e00426-20. doi: 10.1128/mSphere.00426-20. mSphere. 2020. PMID: 32699119 Free PMC article.
-
Quorum sensing by 2-alkyl-4-quinolones in Pseudomonas aeruginosa and other bacterial species.Mol Biosyst. 2008 Sep;4(9):882-8. doi: 10.1039/b803796p. Epub 2008 Jun 30. Mol Biosyst. 2008. PMID: 18704225 Review.
-
The Molecular Architecture of Pseudomonas aeruginosa Quorum-Sensing Inhibitors.Mar Drugs. 2022 Jul 28;20(8):488. doi: 10.3390/md20080488. Mar Drugs. 2022. PMID: 36005489 Free PMC article. Review.
Cited by
-
A. castellanii and P. aeruginosa mutually exacerbate damage to corneal cells during coinfection.Microbiol Spectr. 2024 Jan 11;12(1):e0268323. doi: 10.1128/spectrum.02683-23. Epub 2023 Dec 14. Microbiol Spectr. 2024. PMID: 38095463 Free PMC article.
-
Shifts from cooperative to individual-based predation defense determine microbial predator-prey dynamics.ISME J. 2023 May;17(5):775-785. doi: 10.1038/s41396-023-01381-5. Epub 2023 Feb 28. ISME J. 2023. PMID: 36854789 Free PMC article.
-
Amoebae as training grounds for microbial pathogens.mBio. 2024 Aug 14;15(8):e0082724. doi: 10.1128/mbio.00827-24. Epub 2024 Jul 8. mBio. 2024. PMID: 38975782 Free PMC article. Review.
-
Examining the influence of environmental factors on Acanthamoeba castellanii and Pseudomonas aeruginosa in co-culture.PLoS One. 2024 Jun 24;19(6):e0305973. doi: 10.1371/journal.pone.0305973. eCollection 2024. PLoS One. 2024. PMID: 38913685 Free PMC article.
-
The Epistemology of Bacterial Virulence Factor Characterization.Microorganisms. 2024 Jun 22;12(7):1272. doi: 10.3390/microorganisms12071272. Microorganisms. 2024. PMID: 39065041 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

