Achilles Tendon Ruptures in Middle-Aged Rats Heal Poorly Compared With Those in Young and Old Rats
- PMID: 34851182
- PMCID: PMC8819270
- DOI: 10.1177/03635465211055476
Achilles Tendon Ruptures in Middle-Aged Rats Heal Poorly Compared With Those in Young and Old Rats
Abstract
Background: Achilles tendon ruptures are painful and debilitating injuries and are most common in middle-aged patients. There is a lack of understanding of the underlying causes for increased rupture rates in middle-aged patients and how healing outcomes after a rupture might be affected by patient age. Therefore, the objective of this study was to define age-specific Achilles tendon healing by assessing ankle functional outcomes and Achilles tendon mechanical and histological properties after a rupture using a rat model.
Hypothesis: Rats representing the middle-aged patient population would demonstrate reduced healing capability after an Achilles tendon rupture, as demonstrated by a slower return to baseline ankle functional properties and inferior biomechanical and histological tendon properties.
Study design: Controlled laboratory study.
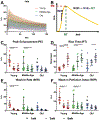
Methods: Fischer 344 rats were categorized by age to represent young, middle-aged, and old patients, and Achilles tendon ruptures were induced in the right hindlimb. Animals were allowed to heal and were euthanized at 3 or 6 weeks after the injury. In vivo functional assays and ultrasound imaging were performed throughout the healing period, and ex vivo tendon mechanical and histological properties were assessed after euthanasia.
Results: Rats representing middle-aged patients displayed reduced healing potential compared with the other age groups, as they demonstrated decreased recovery of in vivo functional and ultrasound assessment parameters and inferior mechanical and histological properties after an Achilles tendon rupture.
Conclusion: These findings may help explain the increased rupture rate observed clinically in middle-aged patients by suggesting that there may be altered tendon responses to daily trauma.
Clinical relevance: The results provide novel data on age-specific healing outcomes after an Achilles tendon rupture, which underscores the importance of considering a patient's age during treatment and expectations for outcomes.
Keywords: Achilles tendon; ankle; biology of tendon; biomechanics of tendon.
Figures



Similar articles
-
Limited Scar Resection for Chronic Achilles Tendon Repair: Use of a Rat Model.Am J Sports Med. 2021 Aug;49(10):2707-2715. doi: 10.1177/03635465211023096. Epub 2021 Jul 1. Am J Sports Med. 2021. PMID: 34197235 Free PMC article.
-
Achilles tendon elastic properties remain decreased in long term after rupture.Knee Surg Sports Traumatol Arthrosc. 2018 Jul;26(7):2080-2087. doi: 10.1007/s00167-017-4791-4. Epub 2017 Nov 16. Knee Surg Sports Traumatol Arthrosc. 2018. PMID: 29147741
-
Evaluation of Elastic Stiffness in Healing Achilles Tendon After Surgical Repair of a Tendon Rupture Using In Vivo Ultrasound Shear Wave Elastography.Med Sci Monit. 2016 Apr 9;22:1186-91. doi: 10.12659/MSM.895674. Med Sci Monit. 2016. PMID: 27072885 Free PMC article.
-
Achilles tendon rupture; assessment of nonoperative treatment.Dan Med J. 2014 Apr;61(4):B4837. Dan Med J. 2014. PMID: 24814601 Review.
-
Outcomes and Complications of Open Versus Minimally Invasive Repair of Acute Achilles Tendon Ruptures: A Systematic Review and Meta-analysis of Randomized Controlled Trials.Am J Sports Med. 2023 Mar;51(3):825-836. doi: 10.1177/03635465211053619. Epub 2021 Dec 15. Am J Sports Med. 2023. PMID: 34908499
Cited by
-
Moderate- and High-Speed Treadmill Running Exercise Have Minimal Impact on Rat Achilles Tendon.J Orthop Res. 2025 Mar;43(3):519-530. doi: 10.1002/jor.26030. Epub 2024 Dec 27. J Orthop Res. 2025. PMID: 39731286 Free PMC article.
-
Plantar flexor deficits following Achilles tendon rupture: A novel small animal dynamometer and detailed instructions.J Biomech. 2022 Dec;145:111393. doi: 10.1016/j.jbiomech.2022.111393. Epub 2022 Nov 21. J Biomech. 2022. PMID: 36442431 Free PMC article.
-
Understanding altered contractile properties in advanced age: insights from a systematic muscle modelling approach.Biomech Model Mechanobiol. 2023 Feb;22(1):309-337. doi: 10.1007/s10237-022-01651-9. Epub 2022 Nov 6. Biomech Model Mechanobiol. 2023. PMID: 36335506 Free PMC article.
-
Novel application of in vivo microdialysis in a rat Achilles tendon acute injury model.J Appl Physiol (1985). 2024 Jan 1;136(1):43-52. doi: 10.1152/japplphysiol.00720.2023. Epub 2023 Nov 16. J Appl Physiol (1985). 2024. PMID: 37969085 Free PMC article.
-
Sixteen weeks of high-speed treadmill running is insufficient to induce Achilles tendinopathy in a rat model.Am J Physiol Cell Physiol. 2025 Jun 1;328(6):C2013-C2022. doi: 10.1152/ajpcell.00186.2025. Epub 2025 May 8. Am J Physiol Cell Physiol. 2025. PMID: 40337916 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

