Case Report: First Confirmed Case of Coinfection of SARS-CoV-2 With Choclo orthohantavirus
- PMID: 34851327
- PMCID: PMC8594034
- DOI: 10.3389/fitd.2021.769330
Case Report: First Confirmed Case of Coinfection of SARS-CoV-2 With Choclo orthohantavirus
Abstract
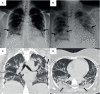
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused a major international public health concern. The World Health Organization (WHO) declared the pandemic of coronavirus disease 2019 (COVID-19) on March 11, 2020. In Panama, the first SARS-CoV-2 infection was confirmed on March 9, 2020, and the first fatal case associated to COVID-19 was reported on March 10. This report presents the case of a 44-year-old female who arrived at the hospital with a respiratory failure, five days after the first fatal COVID-19 case, and who was living in a region where hantavirus pulmonary syndrome cases caused by Choclo orthohantavirus (CHOV), are prevalent. Thus, the clinical personnel set a differential diagnosis to determine a respiratory disease caused by the endemic CHOV or the new pandemic SARS-CoV-2. This case investigation describes the first coinfection by SARS-CoV-2 and CHOV worldwide. PCR detected both viruses during early stages of the disease and the genomic sequences were obtained. The presence of antibodies was determined during the patient's hospitalization. After 23 days at the intensive care unit, the patient survived with no sequelae, and antibodies against CHOV and SARS-CoV-2 were still detectable 12 months after the disease. The detection of the coinfection in this patient highlights the importance, during a pandemic, of complementing the testing and diagnosis of the emergent agent, SARS-CoV-2, with other common endemic respiratory pathogens and other zoonotic pathogens, like CHOV, in regions where they are of public health concern.
Keywords: COVID-19; Choclo orthohantavirus; SARS-CoV-2; case report; coinfection; hantavirus pulmonary syndrome.
Copyright © 2021 Hesse, Nuñez, Salazar, Salinas, Barrera, Chong, Torres, Cumbrera, Olivares, Junco, Matteo, González, Chavarría, Moreno, Góndola, Ábrego, Díaz, Pitti, Franco, Martínez-Montero, Pascale, López-Vergès, Martínez and Armién.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
