Drug Survival of Biologics in Patients With Hidradenitis Suppurativa
- PMID: 34851360
- PMCID: PMC8637394
- DOI: 10.1001/jamadermatol.2021.4805
Drug Survival of Biologics in Patients With Hidradenitis Suppurativa
Abstract
Importance: Biologics are important in treating patients with hidradenitis suppurativa (HS). However, to our knowledge, data on their real-life performance and treatment patterns in HS are limited.
Objective: To examine the drug survival of biologic therapies for HS in a real-world setting.
Design, setting, and participants: This cohort study included all patients with HS between January 1, 2005, and December 31, 2018, who were treated with biologics at the 5 academic hospital clinics where all biologic treatment for HS is conducted in Denmark. Biologics included adalimumab, anakinra, certolizumab pegol, etanercept, golimumab, infliximab, secukinumab, and ustekinumab. Data were analyzed between June 1, 2021, and June 20, 2021.
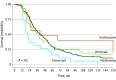
Main outcomes and measures: Drug survival was depicted through Kaplan-Meier curves, and Cox regression models were used to calculate adjusted (age, sex, previous number of biologic treatment series) hazard ratios (aHRs) with 95% CIs for the risk of treatment discontinuation. Switching patterns were visualized through a Sankey diagram.
Results: The study comprised 241 patients (176 women [61.8%]; total of 386 treatment series) with a mean (SD) age of 41.8 (12.6) years at initiation of first biologic therapy. There were a total of 256 (189 [73.8%] biologic naive), 66 (32 [48.5%] biologic naive), 23 (9 [39.1%] biologic naive), and 22 (9 [40.9%] biologic naive) treatment series with adalimumab, infliximab, etanercept, and ustekinumab, respectively. The median time to discontinuation was 36.0 (IQR, 21.9-63.0), 28.7 (IQR, 15.1-62.9), 26.0 (IQR, 16.9-155.9), and 17.9 weeks (IQR, 12.9-41.0) for adalimumab, infliximab, ustekinumab and etanercept, respectively. The risk of drug discontinuation was significantly higher for etanercept compared with adalimumab (aHR, 1.81; 95% CI, 1.16-2.82), infliximab (aHR, 1.77; 95% CI, 1.03-3.05), and ustekinumab (aHR, 2.49; 95% CI, 1.12-5.52), whereas no difference was observed when comparing these 3 therapies with each other. We found no significant differences in drug survival for biologic-naive vs nonnaive treatment series. Increasing C-reactive protein levels (aHR, 1.01; 95% CI, 1.00-1.03) and concomitant antibiotic treatment (aHR, 2.82; 95% CI, 1.36-5.86) were associated with the risk of discontinuing infliximab therapy. Men (aHR, 0.69; 95% CI, 0.51-0.91) had a reduced risk of discontinuing use of adalimumab.
Conclusions and relevance: In this cohort study, drug survival was comparable between adalimumab, infliximab, and ustekinumab but significantly lower for etanercept. There were no differences in drug survival among biologic-naive and nonnaive patients.
Conflict of interest statement
Figures
References
-
- van der Zee HH, de Ruiter L, van den Broecke DG, Dik WA, Laman JD, Prens EP. Elevated levels of tumour necrosis factor (TNF)-α, interleukin (IL)-1β and IL-10 in hidradenitis suppurativa skin: a rationale for targeting TNF-α and IL-1β. Br J Dermatol. 2011;164(6):1292-1298. doi:10.1111/j.1365-2133.2011.10254.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials