Bioavailability of once-daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: the better study
- PMID: 34851532
- PMCID: PMC9285676
- DOI: 10.1111/ctr.14550
Bioavailability of once-daily tacrolimus formulations used in clinical practice in the management of De Novo kidney transplant recipients: the better study
Abstract
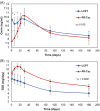
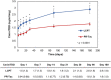
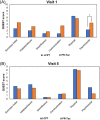
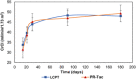
Multicenter, prospective, observational study to compare the relative bioavailability of once-daily tacrolimus formulations in de novo kidney transplant recipients. De novo kidney transplant recipients who started a tacrolimus-based regimen were included 14 days post-transplant and followed up for 6 months. Data from 218 participants were evaluated: 129 in the LCPT group (Envarsus) and 89 in the PR-Tac (Advagraf) group. Patients in the LCPT group exhibited higher relative bioavailability (Cmin /total daily dose [TDD]) vs. PR-Tac (61% increase; P < .001) with similar Cmin and 30% lower TDD levels (P < .0001). The incidence of treatment failure was 3.9% in the LCPT group and 9.0% in the PR-Tac group (P = .117). Study discontinuation rates were 6.2% in the LCPT group and 12.4% in the PR-Tac group (P = .113). Adverse events, renal function and other complications were comparable between groups. The median accumulated dose of tacrolimus in the LCPT group from day 14 to month 6 was 889 mg. Compared to PR-Tac, LCPT showed higher relative bioavailability, similar effectiveness at preventing allograft rejection, comparable effect on renal function, safety, adherence, treatment failure and premature discontinuation rates.
Keywords: bioavailability; clinical practice; pharmacokinetics; renal transplantation; tacrolimus; treatment failure.
© 2021 The Authors. Clinical Transplantation published by John Wiley & Sons Ltd.
Conflict of interest statement
Constantino Fernández has received honoraria from Alexion, Novartis, Astelas, Chiesi, and Biotest. Amado Andres has received honoraria from Chiesi, Astellas, Novartis and Bristol‐Myers‐Squibb. Domingo Hernandez and Veronica López received honoraria from Chiesi. Francesc Moreso received honoraria from Chiesi, Astellas, Novartis and Bristol‐Myers‐Squibb. Edoardo Melilli received honoraria from Chiesi, Astellas, Novartis, Sandoz, Menarini. Gonzalo Gómez has received lecture fees from speaking on behalf of Novartis, and has been paid advisory boards by Alexion. Ana Sánchez Fructuoso has received honoraria from Chiesi. The rest of the authors report no conflict of interest.
Figures
References
-
- Nankivell BJ, PʼNg CH, OʼConnell PJ, Chapman JR. Calcineurin inhibitor nephrotoxicity through the lens of longitudinal histology. Transplantation. 2016;100(8):1723‐1731. - PubMed
-
- Kuypers DRJ, Peeters PC, Sennesael JJ, et al. Improved adherence to tacrolimus oncE‐Daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 2013;95(2):333‐340. - PubMed
-
- Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet. 2004;43(10):623‐653. - PubMed
-
- Prograf Summary of Product characteristics. Available at: https://www.medicines.org.uk/emc/product/6720/smpc. Last accessed: May 2021.
-
- Advagraf Summary of Product characteristics. Available at: https://www.ema.europa.eu/en/documents/product‐information/advagraf‐epar.... Last accessed: May 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

