X-Ray-Induced Modification of the Photophysical Properties of MAPbBr3 Single Crystals
- PMID: 34851625
- PMCID: PMC8678983
- DOI: 10.1021/acsami.1c16072
X-Ray-Induced Modification of the Photophysical Properties of MAPbBr3 Single Crystals
Abstract
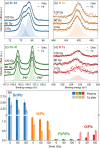
Methylammonium lead tribromide (MAPbBr3) perovskite single crystals demonstrate to be excellent direct X-ray and gamma-ray detectors with outstanding sensitivity and low limit of detection. Despite this, thorough studies on the photophysical effects of exposure to high doses of ionizing radiation on this material are still lacking. In this work, we present our findings regarding the effects of controlled X-ray irradiation on the optoelectronic properties of MAPbBr3 single crystals. Irradiation is carried out in air with an imaging X-ray tube, simulating real-life application in a medical facility. By means of surface photovoltage spectroscopy, we find that X-ray exposure quenches free excitons in the material and introduces new bound excitonic species. Despite this drastic effect, the crystals recover after 1 week of storage in dark and low humidity conditions. By means of X-ray photoelectron spectroscopy, we find that the origin of the new bound excitonic species is the formation of bromine vacancies, leading to local changes in the dielectric response of the material. The recovery effect is attributed to vacancy filling by atmospheric oxygen and water.
Keywords: X-ray photoelectron spectroscopy; excitons; hybrid lead halide perovskites; ionizing radiation; methylammonium lead bromide; surface photovoltage spectroscopy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Surface Engineering of Methylammonium Lead Bromide Perovskite Crystals for Enhanced X-ray Detection.J Phys Chem Lett. 2023 Oct 12;14(40):9136-9144. doi: 10.1021/acs.jpclett.3c02061. Epub 2023 Oct 5. J Phys Chem Lett. 2023. PMID: 37795957 Free PMC article.
-
Structural and Photophysical Properties of Methylammonium Lead Tribromide (MAPbBr3) Single Crystals.Sci Rep. 2017 Oct 20;7(1):13643. doi: 10.1038/s41598-017-13571-1. Sci Rep. 2017. PMID: 29057892 Free PMC article.
-
Identification and Suppression of Point Defects in Bromide Perovskite Single Crystals Enabling Gamma-Ray Spectroscopy.Adv Mater. 2024 Aug;36(35):e2406193. doi: 10.1002/adma.202406193. Epub 2024 Jul 14. Adv Mater. 2024. PMID: 39003617
-
A-site Cation Engineering for Highly Efficient MAPbI3 Single-Crystal X-ray Detector.Angew Chem Int Ed Engl. 2019 Dec 2;58(49):17834-17842. doi: 10.1002/anie.201911281. Epub 2019 Oct 23. Angew Chem Int Ed Engl. 2019. PMID: 31549478 Review.
-
All-Inorganic Lead-Free Perovskite(-Like) Single Crystals: Synthesis, Properties, and Applications.Small Methods. 2021 May;5(5):e2001308. doi: 10.1002/smtd.202001308. Epub 2021 Feb 23. Small Methods. 2021. PMID: 34928084 Review.
Cited by
-
Ionic Field Screening in MAPbBr3 Crystals Revealed from Remnant Sensitivity in X-ray Detection.ACS Phys Chem Au. 2023 May 3;3(4):386-393. doi: 10.1021/acsphyschemau.3c00002. eCollection 2023 Jul 26. ACS Phys Chem Au. 2023. PMID: 37520316 Free PMC article.
-
Structural Modifications due to Bi-Doping in MAPbBr3 Single Crystals and Their Impact on Electronic Transport and Stability.Small. 2024 Dec;20(51):e2407141. doi: 10.1002/smll.202407141. Epub 2024 Oct 9. Small. 2024. PMID: 39380422 Free PMC article.
-
Surface Photovoltage Study of Metal Halide Perovskites Deposited Directly on Crystalline Silicon.ACS Omega. 2023 Feb 24;8(9):8125-8133. doi: 10.1021/acsomega.2c07664. eCollection 2023 Mar 7. ACS Omega. 2023. PMID: 36910941 Free PMC article. Review.
References
-
- Kim H. S.; Lee C. R.; Im J. H.; Lee K. B.; Moehl T.; Marchioro A.; Moon S. J.; Humphry-Baker R.; Yum J. H.; Moser J. E.; Grätzel M.; Park N. G. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591.10.1038/srep00591. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

