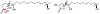Berkeleylactones and a Citreohybriddione Analogue from Penicillium turbatum
- PMID: 34851642
- PMCID: PMC9014984
- DOI: 10.1021/acs.jnatprod.1c00791
Berkeleylactones and a Citreohybriddione Analogue from Penicillium turbatum
Abstract
In 2017 we reported the isolation and characterization of berkeleylactones A-H and A26771B from a coculture of two extremophilic Penicillium sp. isolated from an acid mine waste lake. Berkeleylactone A exhibited potent activity against several strains of multi-drug-resistant Staphylococcus aureus and Bacillus anthracis. A26771B, which is related to the berkeleylactones, also exhibited antibiotic activity. Although the berkeleylactones were novel compounds, A26771B was originally isolated by scientists at Eli Lilly Company from P. turbatum and reported in1977. We recently obtained P. turbatum and grew it in axenic culture. We isolated five new berkeleylactones (2 and 4-7), two berkeley-γ-lactones (8 and 9), and citreohybriddional (10), as well as the known compounds A26771B (1), berkeleylactone E (3), and gliovictin. The structures of the novel compounds were deduced from analysis of spectral data. Compounds 2 and 4 -7 are 16-membered macrolides, while 8 and 9 are γ-lactones that share the hexadecanoic acid skeleton. A26771B (1) and berkeleylactone I (2) were active against several strains of Staphylococcus aureus, including four multi-drug-resistant strains. Berkeleylactone N (8) was active only against Streptococcus pyogenes.
Figures
References
-
- Xu HW; Qin SS; Liu HM Curr. Top. Med. Chem 2014, 14, 21–39. - PubMed
-
- Mcguire JM; Bunch RL; Anderson RC; Boaz HE; Flynn EH; Powell HM; Smith JW Antibiot. Chemother 1952. 2, 281–283. - PubMed
-
- Sekiguchi J; Kuroda H; Yamada Y; Okada H Tetrahedron Lett. 1985, 26, 2341–2342.
-
- Rodphaya D; Sekiguchi J; Yamada YJ Antibiot. 1986, 39, 629–635. - PubMed
-
- Smith CJ; Abbanat D; Bernan VS; Maiese WM; Greenstein M; Jompa J; Tahir A; Ireland CM J. Nat. Prod 2000, 63, 142–145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources