The CD22-IGF2R interaction is a therapeutic target for microglial lysosome dysfunction in Niemann-Pick type C
- PMID: 34851695
- PMCID: PMC9067636
- DOI: 10.1126/scitranslmed.abg2919
The CD22-IGF2R interaction is a therapeutic target for microglial lysosome dysfunction in Niemann-Pick type C
Abstract
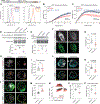
Lysosome dysfunction is a shared feature of rare lysosomal storage diseases and common age-related neurodegenerative diseases. Microglia, the brain-resident macrophages, are particularly vulnerable to lysosome dysfunction because of the phagocytic stress of clearing dying neurons, myelin, and debris. CD22 is a negative regulator of microglial homeostasis in the aging mouse brain, and soluble CD22 (sCD22) is increased in the cerebrospinal fluid of patients with Niemann-Pick type C disease (NPC). However, the role of CD22 in the human brain remains unknown. In contrast to previous findings in mice, here, we show that CD22 is expressed by oligodendrocytes in the human brain and binds to sialic acid–dependent ligands on microglia. Using unbiased genetic and proteomic screens, we identify insulin-like growth factor 2 receptor (IGF2R) as the binding partner of sCD22 on human myeloid cells. Targeted truncation of IGF2R revealed that sCD22 docks near critical mannose 6-phosphate–binding domains, where it disrupts lysosomal protein trafficking. Interfering with the sCD22-IGF2R interaction using CD22 blocking antibodies ameliorated lysosome dysfunction in human NPC1 mutant induced pluripotent stem cell–derived microglia-like cells without harming oligodendrocytes in vitro. These findings reinforce the differences between mouse and human microglia and provide a candidate microglia-directed immunotherapeutic to treat NPC.
Conflict of interest statement
Figures

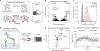


References
-
- Platt FM, d’Azzo A, Davidson BL, Neufeld EF, Tifft CJ, Lysosomal storage diseases. Nat. Rev. Dis. Primers. 4, 27 (2018). - PubMed
-
- Ballabio A, Bonifacino JS, Lysosomes as dynamic regulators of cell and organismal homeostasis. Nat. Rev. Mol. Cell Biol. 21, 101–118 (2020). - PubMed
-
- Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R, Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N. Engl. J. Med. 351, 1972–1977 (2004). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

