VAMP2 and synaptotagmin mobility in chromaffin granule membranes: implications for regulated exocytosis
- PMID: 34851717
- PMCID: PMC9265163
- DOI: 10.1091/mbc.E21-10-0494
VAMP2 and synaptotagmin mobility in chromaffin granule membranes: implications for regulated exocytosis
Abstract
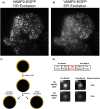
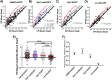
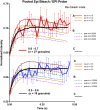
Granule-plasma membrane docking and fusion can only occur when proteins that enable these reactions are present at the granule-plasma membrane contact. Thus, the mobility of granule membrane proteins may influence docking and membrane fusion. We measured the mobility of vesicle associated membrane protein 2 (VAMP2), synaptotagmin 1 (Syt1), and synaptotagmin 7 (Syt7) in chromaffin granule membranes in living chromaffin cells. We used a method that is not limited by standard optical resolution. A bright flash of strongly decaying evanescent field produced by total internal reflection was used to photobleach GFP-labeled proteins in the granule membrane. Fluorescence recovery occurs as unbleached protein in the granule membrane distal from the glass interface diffuses into the more bleached proximal regions, enabling the measurement of diffusion coefficients. We found that VAMP2-EGFP and Syt7-EGFP are mobile with a diffusion coefficient of ∼3 × 10-10 cm2/s. Syt1-EGFP mobility was below the detection limit. Utilizing these diffusion parameters, we estimated the time required for these proteins to arrive at docking and nascent fusion sites to be many tens of milliseconds. Our analyses raise the possibility that the diffusion characteristics of VAMP2 and Syt proteins could be a factor that influences the rate of exocytosis.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

