Short palate, lung, and nasal epithelial clone 1 (SPLUNC1) level determines steroid-resistant airway inflammation in aging
- PMID: 34851736
- PMCID: PMC8759962
- DOI: 10.1152/ajplung.00315.2021
Short palate, lung, and nasal epithelial clone 1 (SPLUNC1) level determines steroid-resistant airway inflammation in aging
Abstract
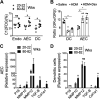
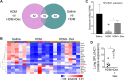
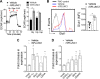
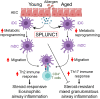
Asthma and its heterogeneity change with age. Increased airspace neutrophil numbers contribute to severe steroid-resistant asthma exacerbation in the elderly, which correlates with the changes seen in adults with asthma. However, whether that resembles the same disease mechanism and pathophysiology in aged and adults is poorly understood. Here, we sought to address the underlying molecular mechanism of steroid-resistant airway inflammation development and response to corticosteroid (Dex) therapy in aged mice. To study the changes in inflammatory mechanism, we used a clinically relevant treatment model of house-dust mite (HDM)-induced allergic asthma and investigated lung adaptive immune response in adult (20-22 wk old) and aged (80-82 wk old) mice. Our result indicates an age-dependent increase in airway hyperresponsiveness (AHR), mixed granulomatous airway inflammation comprising eosinophils and neutrophils, and Th1/Th17 immune response with progressive decrease in frequencies and numbers of HDM-bearing dendritic cells (DC) accumulation in the draining lymph node (DLn) of aged mice as compared with adult mice. RNA-Seq experiments of the aged lung revealed short palate, lung, and nasal epithelial clone 1 (SPLUNC1) as one of the steroid-responsive genes, which progressively declined with age and further by HDM-induced inflammation. Moreover, we found increased glycolytic reprogramming, maturation/activation of DCs, the proliferation of OT-II cells, and Th2 cytokine secretion with recombinant SPLUNC1 (rSPLUNC1) treatment. Our results indicate a novel immunomodulatory role of SPLUNC1 regulating metabolic adaptation/maturation of DC. An age-dependent decline in the SPLUNC1 level may be involved in developing steroid-resistant airway inflammation and asthma heterogeneity.
Keywords: INTRODUCTION; SPLUNC1; aging; airway inflammation; dendritic cells; steroid-resistant asthma.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
SPLUNC1 deficiency enhances airway eosinophilic inflammation in mice.Am J Respir Cell Mol Biol. 2012 Aug;47(2):253-60. doi: 10.1165/rcmb.2012-0064OC. Epub 2012 Apr 12. Am J Respir Cell Mol Biol. 2012. PMID: 22499853 Free PMC article.
-
Immunomodulation of airway epithelium cell activation by mesenchymal stromal cells ameliorates house dust mite-induced airway inflammation in mice.Am J Respir Cell Mol Biol. 2015 Nov;53(5):615-24. doi: 10.1165/rcmb.2014-0431OC. Am J Respir Cell Mol Biol. 2015. PMID: 25789608
-
Dimethyl fumarate abrogates dust mite-induced allergic asthma by altering dendritic cell function.Immun Inflamm Dis. 2019 Sep;7(3):201-213. doi: 10.1002/iid3.262. Epub 2019 Jul 2. Immun Inflamm Dis. 2019. PMID: 31264384 Free PMC article.
-
The Multifunctional Roles of Short Palate, Lung, and Nasal Epithelium Clone 1 in Regulating Airway Surface Liquid and Participating in Airway Host Defense.J Interferon Cytokine Res. 2021 Apr;41(4):139-148. doi: 10.1089/jir.2020.0141. J Interferon Cytokine Res. 2021. PMID: 33885339 Review.
-
Differential short palate, lung, and nasal epithelial clone 1 suppression in eosinophilic and noneosinophilic chronic rhinosinusitis with nasal polyps: implications for pathogenesis and treatment.Curr Opin Allergy Clin Immunol. 2016 Feb;16(1):31-8. doi: 10.1097/ACI.0000000000000228. Curr Opin Allergy Clin Immunol. 2016. PMID: 26658012 Review.
Cited by
-
Aging-Related Mechanisms Contribute to Corticosteroid Insensitivity in Elderly Asthma.Int J Mol Sci. 2023 Mar 28;24(7):6347. doi: 10.3390/ijms24076347. Int J Mol Sci. 2023. PMID: 37047327 Free PMC article. Review.
-
Immunosenescence: A Critical Factor Associated With Organ Injury After Sepsis.Front Immunol. 2022 Jul 18;13:917293. doi: 10.3389/fimmu.2022.917293. eCollection 2022. Front Immunol. 2022. PMID: 35924237 Free PMC article. Review.
-
SPLUNC1 regulates LPS-induced progression of nasopharyngeal carcinoma and proliferation of myeloid-derived suppressor cells.Med Oncol. 2022 Sep 29;39(12):214. doi: 10.1007/s12032-022-01816-7. Med Oncol. 2022. PMID: 36175598
-
UBR5 metabolically reprograms nasopharyngeal carcinoma cells to promote glycolysis and M2 polarization via SPLUNC1 signaling.NPJ Precis Oncol. 2024 Nov 5;8(1):252. doi: 10.1038/s41698-024-00747-y. NPJ Precis Oncol. 2024. PMID: 39501021 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

