Seroprevalence and incidence of hepatitis A in Southeast Asia: A systematic review
- PMID: 34851983
- PMCID: PMC8635355
- DOI: 10.1371/journal.pone.0258659
Seroprevalence and incidence of hepatitis A in Southeast Asia: A systematic review
Abstract
Background: A previous review on hepatitis A virus (HAV) seroprevalence in 2005 categorized Southeast Asia as a low HAV endemicity region. In 2010, the World Health Organization modified this from low to low/medium endemicity, pointing out that these estimates were based on limited evidence. Since then, there has been no attempt to review HAV epidemiology from this region. We conducted a systematic review of literature to collect information on HAV incidence and seroprevalence in select countries in the Southeast Asian region, specifically, The Association of Southeast Asian Nations over the last 20 years.
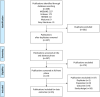
Methodology: This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses guidelines. From the relevant articles, we extracted data and conducted a risk of bias assessment of individual studies.
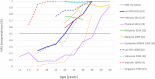
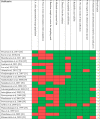
Results: The search yielded 22 and 13 publications on HAV seroprevalence and incidence, respectively. Overall, our findings point to a very low HAV endemicity profile in Thailand and Singapore and evidence of a shift towards low HAV endemicity in Indonesia, Lao People's Democratic Republic, Malaysia, the Philippines, and Vietnam. Only Singapore, Thailand, Malaysia, and the Philippines have existing HAV disease surveillance and reported incidence rates below 1 per 100,000. Several outbreaks with varying magnitude documented in the region provide insights into the evolving epidemiology of HAV in the region. Risk of bias assessment of studies revealed that the individual studies were of low to medium risk.
Conclusions/significance: The available HAV endemicity profiles in Southeast Asian countries, aside from Thailand, are limited and outdated, but suggest an endemicity shift in the region that is not fully documented yet. These findings highlight the need to update information on HAV epidemiology through strengthening of disease surveillance mechanisms to confirm the shift in HAV endemicity in the region.
Conflict of interest statement
GHS, DS, KL, PB, ST, AS and SB are employees of the GSK group of companies. GHS and DS hold shares in the GSK group of companies. YP was supported by The Center of Excellence in Clinical Virology, Chulalongkorn University. Authors declare no other financial and non-financial relationships and activities. GlaxoSmithKline Biologicals SA funded this review and all costs associated with its development and publication. All authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. We declare that HavrixTM, an inactivated hepatitis A vaccine, is a trademark of the GSK group of companies that is marketed in several countries in Southeast Asia under the scope of this review. There are no additional patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- World Health Organization. WHO position paper on hepatitis A vaccines–June 2012. Weekly epidemiological record. 2012;No. 28-29(87):261–76. - PubMed
-
- World Health Organization. WHO immunological basis for immunization series: module 18: hepatitis A. 2019. - PubMed
-
- Centers for Disease Control and Prevention. Hepatitis A. In Epidemiology and Prevention of Vaccine-Preventable Diseases. 13 ed. Hamborsky J KA, Wolfe S, editor. Washington D.C.: Washington D.C. Public Health Foundation; 2015.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

