Impact of Bridging Chemotherapy on Clinical Outcomes of CD19-Specific CAR T Cell Therapy in Children/Young Adults with Relapsed/Refractory B Cell Acute Lymphoblastic Leukemia
- PMID: 34852305
- PMCID: PMC9361393
- DOI: 10.1016/j.jtct.2021.11.014
Impact of Bridging Chemotherapy on Clinical Outcomes of CD19-Specific CAR T Cell Therapy in Children/Young Adults with Relapsed/Refractory B Cell Acute Lymphoblastic Leukemia
Abstract
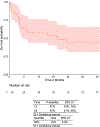
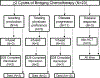
Chimeric antigen receptor (CAR) T cells achieve response and durable remission in patients with relapsed/refractory (R/R) B cell malignancies. Following collection of patient T cells, chemotherapy ("bridging chemotherapy") is utilized during the manufacture of CAR T cells. However, the optimal bridging chemotherapy has yet to be defined. Our objective in this study was to report clinical outcomes following bridging chemotherapy in a cohort of pediatric/young adult patients with R/R B cell acute lymphoblastic leukemia (B-ALL) treated with CAR T cell therapy. This retrospective study included patients enrolled on clinical trial NCT01860937 or referred to Memorial Sloan Kettering Cancer Center for commercial CAR T cell therapy (tisagenlecleucel). Bridging chemotherapy (given after T cell collection and before CAR T cell infusion) was defined as high intensity if myelosuppression was expected for >7 days. Outcome comparison analyses were performed in high-intensity versus low-intensity bridging chemotherapy, 1 cycle versus ≥2 cycles of bridging chemotherapy, disease burden at the start of bridging chemotherapy, disease burden at the start of bridging chemotherapy with chemotherapy intensity, tumor debulking by bridging chemotherapy, and disease burden pre-lymphodepleting chemotherapy (LDC) for CAR T cell treatment. The outcomes of this analysis showed that the incidence of grade ≥3 infection was significantly higher (94% versus 56%; P = .019) and overall survival (OS) was significantly lower (hazard ratio, 3.73; 95% confidence interval, 1.39 to 9.97; P = .006) in patients who received ≥2 cycles versus 1 cycle of bridging chemotherapy. No difference in incidence was found for cytokine release syndrome (P > .99) or neurotoxicity/immune effector cell-associated neurotoxicity syndrome (P = .70). Disease burden at the start of bridging chemotherapy, disease burden prior to LDC, and tumor debulking by bridging chemotherapy also did not significantly affect outcomes after CAR T cell therapy in this cohort. In this study, patients receiving ≥2 cycles of bridging chemotherapy had higher rates of infection and lower OS but no difference in CAR-specific toxicity. Clinicians should carefully consider the use of additional cycles of chemotherapy during the bridging period as it delays treatment with CAR T cells and increases the risk of infectious complications. © 2021 American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc.
Keywords: B cell acute lymphoblastic leukemia; Bridging chemotherapy; CAR T cell therapy; CD19 antigen; Immunotherapy; Tisagenlecleucel.
Copyright © 2021 The American Society for Transplantation and Cellular Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
FINANCIAL DISCLOSURE STATEMENT:
KP discloses patent or royalties (Neximmune, Inc.). JHP discloses consulting or advisory roles (Adaptive Biotechnologies; Amgen; Kite Pharma; Novartis; Pfizer) and research funding (Genentech/Roche; Juno Therapeutics). JJB discloses consulting or advisory roles (Avrobio; Advanced Clinical; Bluerock; Omeros; Takeda; Race Oncology). NAK discloses equity interest (Amgen; Johnson and Johnson; Merck; Pfizer) and research funding (Jazz Pharmaceuticals). KJC discloses consulting or advisory role (Novartis; Mesoblast) and research funding (Juno Therapeutics; Novartis; Celegene). The remaining authors declare no conflicts of interest relevant to this manuscript.
Figures


Similar articles
-
[Long-term follow-up of humanized and murine CD19 CAR-T-cell therapy for B-cell acute lymphoblastic leukemia].Zhonghua Xue Ye Xue Za Zhi. 2023 Oct 14;44(10):793-799. doi: 10.3760/cma.j.issn.0253-2727.2023.10.001. Zhonghua Xue Ye Xue Za Zhi. 2023. PMID: 38049329 Free PMC article. Chinese.
-
The Choice of Either Conventional Chemotherapy or Inotuzumab Ozogamicin as Bridging Regimen Does Not Appear To Impact Clinical Response to CD19-Directed CAR-T Therapy in Pediatric B-ALL.Transplant Cell Ther. 2023 May;29(5):311.e1-311.e7. doi: 10.1016/j.jtct.2023.02.012. Epub 2023 Feb 19. Transplant Cell Ther. 2023. PMID: 36809824
-
Impact of High Disease Burden on Survival in Pediatric Patients with B-ALL Treated with Tisagenlecleucel.Transplant Cell Ther. 2022 Feb;28(2):73.e1-73.e9. doi: 10.1016/j.jtct.2021.11.019. Epub 2021 Dec 4. Transplant Cell Ther. 2022. PMID: 34875402 Free PMC article.
-
Systematic Review and Meta-analysis of CD19-Specific CAR-T Cell Therapy in Relapsed/Refractory Acute Lymphoblastic Leukemia in the Pediatric and Young Adult Population: Safety and Efficacy Outcomes.Clin Lymphoma Myeloma Leuk. 2021 Apr;21(4):e334-e347. doi: 10.1016/j.clml.2020.12.010. Epub 2020 Dec 17. Clin Lymphoma Myeloma Leuk. 2021. PMID: 33573914
-
INSPIRED Symposium Part 4A: Access to CAR T Cell Therapy in Unique Populations with B Cell Acute Lymphoblastic Leukemia.Transplant Cell Ther. 2024 Jan;30(1):56-70. doi: 10.1016/j.jtct.2023.10.005. Epub 2023 Oct 10. Transplant Cell Ther. 2024. PMID: 37821078 Review.
Cited by
-
Long-term response to autologous anti-CD19 chimeric antigen receptor T cells in relapsed or refractory B cell acute lymphoblastic leukemia: a systematic review and meta-analysis.Cancer Gene Ther. 2023 Jun;30(6):845-854. doi: 10.1038/s41417-023-00593-3. Epub 2023 Feb 7. Cancer Gene Ther. 2023. PMID: 36750666 Free PMC article.
-
How I use risk factors for success or failure of CD19 CAR T cells to guide management of children and AYA with B-cell ALL.Blood. 2023 Mar 16;141(11):1251-1264. doi: 10.1182/blood.2022016937. Blood. 2023. PMID: 36416729 Free PMC article. Review.
-
A Multicenter Cohort Study on Children Suffering from Acute Lymphoblastic Leukemia: Effects of Obesity on Mortality.Comput Math Methods Med. 2022 Jul 5;2022:4880151. doi: 10.1155/2022/4880151. eCollection 2022. Comput Math Methods Med. 2022. Retraction in: Comput Math Methods Med. 2023 Jul 19;2023:9893730. doi: 10.1155/2023/9893730. PMID: 35836926 Free PMC article. Retracted.
-
Multinational retrospective analysis of bridging therapy prior to chimeric antigen receptor t cells for relapsed/refractory acute lymphoblastic leukemia in children and young adults.J Hematol Oncol. 2025 Jan 17;18(1):8. doi: 10.1186/s13045-024-01659-x. J Hematol Oncol. 2025. PMID: 39825416 Free PMC article.
-
Approaches for bridging therapy prior to chimeric antigen receptor T cells for relapsed/refractory acute lymphoblastic B-lineage leukemia in children and young adults.Haematologica. 2024 Dec 1;109(12):3892-3903. doi: 10.3324/haematol.2023.283780. Haematologica. 2024. PMID: 38356450 Free PMC article. Review.
References
-
- Reismüller B, Peters C, Dworzak MN, et al. Outcome of children and adolescents with a second or third relapse of acute lymphoblastic leukemia (ALL): a population-based analysis of the Austrian ALL-BFM (Berlin-Frankfurt-Münster) study group. J Pediatr Hematol Oncol. 2013;35:e200–204. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

