Anti-Galectin-2 Antibody Treatment Reduces Atherosclerotic Plaque Size and Alters Macrophage Polarity
- PMID: 34852377
- PMCID: PMC9251707
- DOI: 10.1055/a-1711-1055
Anti-Galectin-2 Antibody Treatment Reduces Atherosclerotic Plaque Size and Alters Macrophage Polarity
Abstract
Background: Galectins have numerous cellular functions in immunity and inflammation. Short-term galectin-2 (Gal-2) blockade in ischemia-induced arteriogenesis shifts macrophages to an anti-inflammatory phenotype and improves perfusion. Gal-2 may also affect other macrophage-related cardiovascular diseases.
Objectives: This study aims to elucidate the effects of Gal-2 inhibition in atherosclerosis.
Methods: ApoE -/- mice were given a high-cholesterol diet (HCD) for 12 weeks. After 6 weeks of HCD, intermediate atherosclerotic plaques were present. To study the effects of anti-Gal-2 nanobody treatment on the progression of existing atherosclerosis, treatment with two llama-derived anti-Gal-2 nanobodies (clones 2H8 and 2C10), or vehicle was given for the remaining 6 weeks.
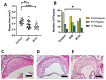
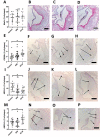
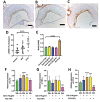
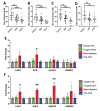
Results: Gal-2 inhibition reduced the progression of existing atherosclerosis. Atherosclerotic plaque area in the aortic root was decreased, especially so in mice treated with 2C10 nanobodies. This clone showed reduced atherosclerosis severity as reflected by a decrease in fibrous cap atheromas in addition to decreases in plaque size.The number of plaque resident macrophages was unchanged; however, there was a significant increase in the fraction of CD206+ macrophages. 2C10 treatment also increased plaque α-smooth muscle content, and Gal-2 may have a role in modulating the inflammatory status of smooth muscle cells. Remarkably, both treatments reduced serum cholesterol concentrations including reductions in very low-density lipoprotein, low-density lipoprotein, and high-density lipoprotein while triglyceride concentrations were unchanged.
Conclusion: Prolonged and frequent treatment with anti-Gal-2 nanobodies reduced plaque size, slowed plaque progression, and modified the phenotype of plaque macrophages toward an anti-inflammatory profile. These results hold promise for future macrophage modulating therapeutic interventions that promote arteriogenesis and reduce atherosclerosis.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Conflict of interest statement
M.V. reports having served as advisor for FMC, Amgen, Medice, Otsuka, Vifor, and Kyowa Kirin. Research support from FMC, Amgen, and Vifor. No other conflicts of interest to report.
Figures
Similar articles
-
Stimulation of Collateral Vessel Growth by Inhibition of Galectin 2 in Mice Using a Single-Domain Llama-Derived Antibody.J Am Heart Assoc. 2019 Oct 15;8(20):e012806. doi: 10.1161/JAHA.119.012806. Epub 2019 Oct 9. J Am Heart Assoc. 2019. PMID: 31594443 Free PMC article.
-
Palmitoylethanolamide Promotes a Proresolving Macrophage Phenotype and Attenuates Atherosclerotic Plaque Formation.Arterioscler Thromb Vasc Biol. 2018 Nov;38(11):2562-2575. doi: 10.1161/ATVBAHA.118.311185. Arterioscler Thromb Vasc Biol. 2018. PMID: 30354245
-
Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice.Glycobiology. 2013 Jun;23(6):654-63. doi: 10.1093/glycob/cwt006. Epub 2013 Feb 19. Glycobiology. 2013. PMID: 23426722 Free PMC article.
-
New Targets in Atherosclerosis: Vascular Smooth Muscle Cell Plasticity and Macrophage Polarity.Clin Ther. 2023 Nov;45(11):1047-1054. doi: 10.1016/j.clinthera.2023.08.015. Epub 2023 Sep 13. Clin Ther. 2023. PMID: 37709601 Review.
-
Macrophage-, Dendritic-, Smooth Muscle-, Endothelium-, and Stem Cells-Derived Foam Cells in Atherosclerosis.Int J Mol Sci. 2022 Nov 16;23(22):14154. doi: 10.3390/ijms232214154. Int J Mol Sci. 2022. PMID: 36430636 Free PMC article. Review.
Cited by
-
Galectokines: The Promiscuous Relationship between Galectins and Cytokines.Biomolecules. 2022 Sep 13;12(9):1286. doi: 10.3390/biom12091286. Biomolecules. 2022. PMID: 36139125 Free PMC article. Review.
-
Effects of Transglutaminase and Heat Treatment on the Structure and Gelation Properties of Camel Casein Protein.Foods. 2025 May 7;14(9):1644. doi: 10.3390/foods14091644. Foods. 2025. PMID: 40361726 Free PMC article.
-
Galectin-2 in Health and Diseases.Int J Mol Sci. 2022 Dec 25;24(1):341. doi: 10.3390/ijms24010341. Int J Mol Sci. 2022. PMID: 36613785 Free PMC article. Review.
-
Galectin functions in cancer-associated inflammation and thrombosis.Front Cardiovasc Med. 2023 Feb 17;10:1052959. doi: 10.3389/fcvm.2023.1052959. eCollection 2023. Front Cardiovasc Med. 2023. PMID: 36873388 Free PMC article. Review.
-
Single domain antibody: Development and application in biotechnology and biopharma.Immunol Rev. 2024 Nov;328(1):98-112. doi: 10.1111/imr.13381. Epub 2024 Aug 21. Immunol Rev. 2024. PMID: 39166870 Free PMC article. Review.
References
-
- Gisterå A, Hansson G K. The immunology of atherosclerosis. Nat Rev Nephrol. 2017;13(06):368–380. - PubMed
-
- Loser K, Sturm A, Voskort M. Galectin-2 suppresses contact allergy by inducing apoptosis in activated CD8+ T cells. J Immunol. 2009;182(09):5419–5429. - PubMed
-
- Rabinovich G A, Toscano M A. Turning ‘sweet’ on immunity: galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9(05):338–352. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

