Use of intravenous lidocaine for dose reduction of propofol in paediatric colonoscopy patients: a randomised placebo-controlled study
- PMID: 34852767
- PMCID: PMC8638197
- DOI: 10.1186/s12871-021-01525-0
Use of intravenous lidocaine for dose reduction of propofol in paediatric colonoscopy patients: a randomised placebo-controlled study
Abstract
Background: Propofol, a widely used sedative in endoscopic procedures, sometimes causes cardiopulmonary complications. Intravenous lidocaine can diminish visceral pain and decrease the dose of propofol. The purpose of this study was to assess the efficacy and safety of intravenous lidocaine in reducing propofol dosage during paediatric colonoscopy.
Methods: Forty children who underwent colonoscopy were divided into two groups. Lidocaine hydrochloride (1.5 mg/kg induction and 2 mg/kg/h maintenance) was given intravenously to the lidocaine group, and the same amount of saline was given to the control group after they received lidocaine induction. Propofol initial plasma concentration of 5 μg/mL was targeted, and the procedure was performed after the bispectral index value reached 55. The primary outcome was propofol requirement.
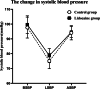
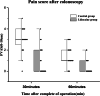
Results: The propofol requirement in the lidocaine group was decreased by 35.5% (128.6 ± 30.4 mg vs. 199.4 ± 57.6 mg; p < 0.001; 95%CI: - 100.60, - 41.02). The incidence of involuntary body movements was significantly lower in the lidocaine group (p = 0.028; OR = 0.17; 95%CI: 0.03, 0.92). The awakening time (p < 0.001; 95%CI: - 7.67, - 5.13) and recovery times (p < 0.001; 95%CI: - 7.45, - 4.35) were significantly lower in the lidocaine group. Pain was significantly less at 30 min and 60 min after the procedure in the lidocaine group (0 [0-4] vs. 3 [0-5], p < 0. 001; 0 [0-2] vs. 1 [0-3], p = 0.001). There was no difference in the incidence of bradycardia, hypotension, or hypoxia between the two groups.
Conclusions: For colonoscopy procedures in paediatric patients, intravenous lidocaine reduces the amount of propofol needed, provides better sedation and postprocedural pain management, as well as a reduction in recovery time.
Trial registration: The trial was registered on November 6, 2020 at China Clinical Trials Registration Center ( www.chictr.org.cn ) ref.: ChiCTR 2,000,039,706.
Keywords: Adverse events; Children; Colonoscopy; Lidocaine; Propofol.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Intravenous infusion of lidocaine significantly reduces propofol dose for colonoscopy: a randomised placebo-controlled study.Br J Anaesth. 2018 Nov;121(5):1059-1064. doi: 10.1016/j.bja.2018.06.019. Epub 2018 Aug 1. Br J Anaesth. 2018. PMID: 30336850 Clinical Trial.
-
The propofol-sparing effect of intravenous lidocaine in elderly patients undergoing colonoscopy: a randomized, double-blinded, controlled study.BMC Anesthesiol. 2020 May 30;20(1):132. doi: 10.1186/s12871-020-01049-z. BMC Anesthesiol. 2020. PMID: 32473649 Free PMC article. Clinical Trial.
-
Intravenous lidocaine with propofol-based sedation for colonoscopy: a systematic review and meta-analysis with trial sequential analysis.Anaesthesia. 2025 Jun;80(6):694-703. doi: 10.1111/anae.16563. Epub 2025 Mar 18. Anaesthesia. 2025. PMID: 40102176
-
Intravenous lidocaine decreased oxygen-desaturation episodes induced by propofol-based sedation for gastrointestinal endoscopy procedures: a prospective, randomized, controlled trial.BMC Anesthesiol. 2025 Jan 11;25(1):27. doi: 10.1186/s12871-025-02890-w. BMC Anesthesiol. 2025. PMID: 39799289 Free PMC article. Clinical Trial.
-
Efficacy of lidocaine on preventing incidence and severity of pain associated with propofol using in pediatric patients: A PRISMA-compliant meta-analysis of randomized controlled trials.Medicine (Baltimore). 2017 Mar;96(11):e6320. doi: 10.1097/MD.0000000000006320. Medicine (Baltimore). 2017. PMID: 28296748 Free PMC article.
Cited by
-
Intravenous Lignocaine as an Adjunct to Propofol Based Sedation in Colonoscopy: A Prospective, Observational Study.Ann Afr Med. 2025 Apr 1;24(2):225-230. doi: 10.4103/aam.aam_84_24. Epub 2025 Feb 21. Ann Afr Med. 2025. PMID: 39981869 Free PMC article.
-
The Effect of Preoperative Combined with Intravenous Lidocaine and Ketamine vs. Intravenous Ketamine on Pediatric Patients Undergoing Upper Gastrointestinal Endoscopy.Anesth Pain Med. 2023 Mar 10;13(2):e130991. doi: 10.5812/aapm-130991. eCollection 2023 Apr. Anesth Pain Med. 2023. PMID: 37645009 Free PMC article.
-
Virtual reality as a strategy for intra-operatory anxiolysis and pharmacological sparing in patients undergoing breast surgeries: The V-RAPS randomized controlled trial protocol.PLoS One. 2025 Jul 7;20(7):e0327555. doi: 10.1371/journal.pone.0327555. eCollection 2025. PLoS One. 2025. PMID: 40622954 Free PMC article.
References
-
- Kauffman RE, Jr, WB. Berlin CM, Blumer JL, Temple AR. Guideline for monitoring and Management of Pediatric Patients during and after Sedation for diagnostic and therapeutic procedures. Pediatrics. 1992;89(6):1110–1115. - PubMed
-
- Berzin TM, Sanaka S, Barnett SR, Sundar E, Sepe PS, Jakubowski M, Pleskow DK, Chuttani R, Sawhney MS. A prospective assessment of sedation-related adverse events and patient and endoscopist satisfaction in ERCP with anesthesiologist-administered sedation. Gastrointest Endosc. 2011;73(4):710–717. doi: 10.1016/j.gie.2010.12.011. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

