Improving the understanding of how patients with non-dystrophic myotonia are selected for myotonia treatment with mexiletine (NaMuscla): outcomes of treatment impact using a European Delphi panel
- PMID: 34852780
- PMCID: PMC8633892
- DOI: 10.1186/s12883-021-02491-3
Improving the understanding of how patients with non-dystrophic myotonia are selected for myotonia treatment with mexiletine (NaMuscla): outcomes of treatment impact using a European Delphi panel
Abstract
Background: Non-dystrophic myotonias (NDMs) comprise muscle chloride and sodium channelopathies due to genetic defects of the CLCN1- and SCN4A-channels. No licensed antimyotonic treatment has been available until approval of mexiletine (NaMuscla®) for adult patients by the EMA in December 2018. This Delphi panel aimed to understand how outcomes of the pivotal phase III Mexiletine study (MYOMEX) translate to real world practice and investigate health resource use, quality of life and the natural history of NDM to support economic modelling and facilitate patient access.
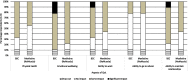
Methods: Nine clinical experts in treating NDM took part in a two-round Delphi panel. Their knowledge of NDM and previous use of mexiletine as an off-label treatment prior to NaMuscla's approval ensured they could provide both qualitative context and quantitative estimates to support economic modelling comparing mexiletine (NaMuscla) to best supportive care. Consensus in four key areas was sought: healthcare resource utilization (HRU), treatment with mexiletine (NaMuscla), patient quality of life (QoL), and the natural history of disease. Concept questions were also asked, considering perceptions on the feasibility of mapping the validated Individualized Neuromuscular Quality of Life (INQoL) instrument to the generic EQ-5D™, and the potential impact on caregiver QoL.
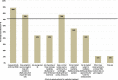
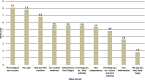
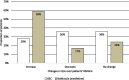
Results: Consensus was achieved for key questions including the average long-term dosage of mexiletine (NaMuscla) in practice, the criteria for eligibility of myotonia treatment, the clinical importance of QoL outcomes in MYOMEX, the higher proportion of patients with increased QoL, and the reduction in the need for mental health resources for patients receiving mexiletine (NaMuscla). While consensus was not achieved for other questions, the results demonstrated that most experts felt mexiletine (NaMuscla) reduced the need for HRU and was expected to improve QoL. The QoL mapping exercise suggested that it is feasible to map domains of INQoL to EQ-5D. Points of interest for future research were identified, including that mexiletine (NaMuscla) may slow the annual decrease in QoL of patients over their lifetime, and a significant negative impact on QoL for some caregivers.
Conclusions: This project successfully provided data from an informed group of clinical experts, complementing the currently available clinical trial data for mexiletine (NaMuscla) to support patient access decisions.
Keywords: Delphi panel; EQ-5D; Healthcare resource utilisation; INQoL; Mexiletine (NaMuscla); Non-dystrophic myotonia; Quality of life.
© 2021. The Author(s).
Conflict of interest statement
AC, MS and LW are employees of BresMed Health Solutions Ltd. BresMed Health Solutions Ltd. was commissioned and received funding from Lupin Atlantis Holdings SA to conduct this study.
CE, AO, SS, AZW are employees of subsidiaries of Lupin Atlantis Holdings SA.
HL is an employee of AGH Partners Ltd., and works as a consultant for Lupin Ltd.
CSG is a Professor and neurologist, specialising in myotonic disorders, employed by the St. Josef-Hospital in Bochum, Germany. CSG worked as an independent clinical advisor on this study and received a financial compensation from Lupin Atlantis Holdings SA for her review of study materials at the design stage of the Delphi panel; she did not receive a financial compensation for work related to the development of this manuscript.
All Delphi panellists received a financial compensation from Lupin Atlantis Holdings SA, in line with the applicable national standards, for their time to participate in this study.
Figures
Similar articles
-
Expert Insights from a Delphi-driven Neurologists' Panel: Real-world Mexiletine use in Patients with Myotonic Disorders in Italy.J Neuromuscul Dis. 2024;11(2):411-423. doi: 10.3233/JND-230115. J Neuromuscul Dis. 2024. PMID: 38306059 Free PMC article.
-
Mexiletine for symptoms and signs of myotonia in nondystrophic myotonia: a randomized controlled trial.JAMA. 2012 Oct 3;308(13):1357-65. doi: 10.1001/jama.2012.12607. JAMA. 2012. PMID: 23032552 Free PMC article. Clinical Trial.
-
Non-dystrophic myotonia: 2-year clinical and patient reported outcomes.Muscle Nerve. 2022 Aug;66(2):148-158. doi: 10.1002/mus.27649. Epub 2022 Jun 16. Muscle Nerve. 2022. PMID: 35644941 Free PMC article.
-
Pharmacological therapy of non-dystrophic myotonias.Acta Myol. 2025 Mar;44(1):23-27. doi: 10.36185/2532-1900-1026. Acta Myol. 2025. PMID: 40183437 Free PMC article. Review.
-
Blockers of Skeletal Muscle Nav1.4 Channels: From Therapy of Myotonic Syndrome to Molecular Determinants of Pharmacological Action and Back.Int J Mol Sci. 2023 Jan 3;24(1):857. doi: 10.3390/ijms24010857. Int J Mol Sci. 2023. PMID: 36614292 Free PMC article. Review.
Cited by
-
Clinical and molecular characterization of myotonia congenita using whole-exome sequencing in Egyptian patients.Mol Biol Rep. 2024 Jun 15;51(1):766. doi: 10.1007/s11033-024-09646-8. Mol Biol Rep. 2024. PMID: 38877370
-
Preference-based utility weights for the Individualized Neuromuscular Quality of Life Questionnaire (INQoL), with a focus on non-dystrophic myotonia (NDM).Eur J Health Econ. 2024 Nov;25(8):1461-1469. doi: 10.1007/s10198-024-01674-2. Epub 2024 Feb 28. Eur J Health Econ. 2024. PMID: 38416296 Free PMC article.
-
Expert Insights from a Delphi-driven Neurologists' Panel: Real-world Mexiletine use in Patients with Myotonic Disorders in Italy.J Neuromuscul Dis. 2024;11(2):411-423. doi: 10.3233/JND-230115. J Neuromuscul Dis. 2024. PMID: 38306059 Free PMC article.
References
-
- European Medicines Agency . Orphan maintenance assessment report - NaMuscla (mexiletine hydrochloride) 2018.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

