A nuanced role of the small loop of hepatitis B virus small envelope protein in virion morphogenesis and secretion
- PMID: 34852809
- PMCID: PMC8638367
- DOI: 10.1186/s12929-021-00780-0
A nuanced role of the small loop of hepatitis B virus small envelope protein in virion morphogenesis and secretion
Abstract
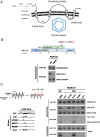
Background: The virion secretion mechanism of human hepatitis B virus (HBV) remains to be investigated. In our current study, we characterized a reverse transcriptase mutant, which changed from the YMDD motif to YMHA. We noted that this mutant YMHA secreted no virions in the medium. Because of the overlapping open reading frame (ORF) between the polymerase and the envelope genes, the lack of virion secretion is likely due to corresponding concurrent mutations in a small loop of the envelope protein (HBsAg, HBV surface antigen). In literature, small loop mutations are thought to affect virion secretion of hepatitis delta virus (HDV), but not HBV.
Methods: Here, we revisited the relationship between the small loop and virion secretion by site-directed mutagenesis and native agarose gel electrophoresis.
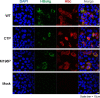
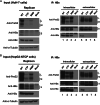
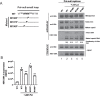
Results: A proline substitution at residue 196 or 198 in the small loop blocked both HBV genome-containing and genome-free virion secretion, but not the secretion of 22-nm HBsAg subviral particles. Surprisingly, a leucine substitution at residue 196 enhanced genome-containing virion secretion. It is also intriguing that a proline-197, sandwiched by residue 196 and 198, exhibited no apparent defect in secreted virions, with or without containing an HBV genome. By complementation assay, we demonstrated that the wild type small envelope protein alone is sufficient to rescue the virion secretion defect of a small loop mutant M198P.
Conclusions: The effect of the small loop mutation of HBV small envelope protein on virion secretion is position-dependent. It warrants further investigation how the small loop of HBsAg plays a subtle role in HBV morphogenesis and secretion of virions with or without containing an HBV genome.
Keywords: Envelope protein; HBV; Hepatitis B virus; Proline substitution; Small loop; Virion secretion.
© 2021. The Author(s).
Conflict of interest statement
We declare no competing financial or non-financial interests. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Shih C, Yang CC, Choijilsuren G, Chang CH, Liou AT. Hepatitis B virus. Trends Microbiol. 2018;26(4):386–387. - PubMed
-
- Shih C, Chou SF, Yang CC, Huang JY, Choijilsuren G, Jhou RS. Control and eradication strategies of hepatitis B virus. Trends Microbiol. 2016;24(9):739–749. - PubMed
-
- Sureau C, Negro F. The hepatitis delta virus: replication and pathogenesis. J Hepatol. 2016;64:S102–S116. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

