BUB1 drives the occurrence and development of bladder cancer by mediating the STAT3 signaling pathway
- PMID: 34852826
- PMCID: PMC8638147
- DOI: 10.1186/s13046-021-02179-z
BUB1 drives the occurrence and development of bladder cancer by mediating the STAT3 signaling pathway
Abstract
Background: The incidence of bladder urothelial carcinoma (UC), a common malignancy of the urinary tract, is approximately three times higher in men than in women. High expression of the mitotic kinase BUB1 is associated with the occurrence and development of several cancers, although the relationship between BUB1 and bladder tumorigenesis remains unclear.
Methods: Using a microarray approach, we found increased BUB1 expression in human BCa. The association between BUB1 and STAT3 phosphorylation was determined through molecular and cell biological methods. We evaluated the impact of pharmacologic inhibition of BUB1 kinase activity on proliferation and BCa progression in vitro and in vivo.
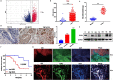
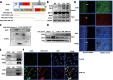
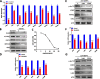
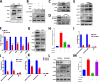
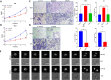
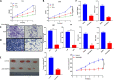
Results: In this study, we found that BUB1 expression was increased in human bladder cancer (BCa). We further identified through a series of molecular and cell biological approaches that BUB1 interacted directly with STAT3 and mediated the phosphorylation of STAT3 at Ser727. In addition, the findings that pharmacologic inhibition of BUB1 kinase activity significantly suppressed BCa cell proliferation and the progression of bladder cancer in vitro and in vivo were further verified. Finally, we found that the BUB1/STAT3 complex promoted the transcription of STAT3 target genes and that depletion of BUB1 and mutation of the BUB1 kinase domain abrogated this transcriptional activity, further highlighting the critical role of kinase activity in the activation of STAT3 target genes. A pharmacological inhibitor of BUB1 (2OH-BNPP1) was able to significantly inhibit the growth of BCa cell xenografts.
Conclusion: This study showed that the BUB1 kinase drives the progression and proliferation of BCa by regulating the transcriptional activation of STAT3 signaling and may be an attractive candidate for therapeutic targeting in BCa.
Keywords: BUB1; Bladder cancer; Phosphorylation; Proliferation; STAT3.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no potential conflicts of interest.
Figures


Similar articles
-
The kinase activity of the Ser/Thr kinase BUB1 promotes TGF-β signaling.Sci Signal. 2015 Jan 6;8(358):ra1. doi: 10.1126/scisignal.2005379. Sci Signal. 2015. PMID: 25564677 Free PMC article.
-
Tanshinone IIA Inhibits Epithelial-Mesenchymal Transition in Bladder Cancer Cells via Modulation of STAT3-CCL2 Signaling.Int J Mol Sci. 2017 Jul 25;18(8):1616. doi: 10.3390/ijms18081616. Int J Mol Sci. 2017. PMID: 28757590 Free PMC article.
-
Berberine suppresses bladder cancer cell proliferation by inhibiting JAK1-STAT3 signaling via upregulation of miR-17-5p.Biochem Pharmacol. 2021 Jun;188:114575. doi: 10.1016/j.bcp.2021.114575. Epub 2021 Apr 20. Biochem Pharmacol. 2021. PMID: 33887260
-
Aurora B hyperactivation by Bub1 overexpression promotes chromosome missegregation.Cell Cycle. 2011 Nov 1;10(21):3645-51. doi: 10.4161/cc.10.21.18156. Epub 2011 Nov 1. Cell Cycle. 2011. PMID: 22033440 Free PMC article. Review.
-
The Sirenic Links between Diabetes, Obesity, and Bladder Cancer.Int J Mol Sci. 2021 Oct 15;22(20):11150. doi: 10.3390/ijms222011150. Int J Mol Sci. 2021. PMID: 34681810 Free PMC article. Review.
Cited by
-
The key regulator circPDE3B promotes arsenic-induced bladder carcinogenesis by affecting STAT3 and NF-κB stability.Cell Biol Toxicol. 2025 May 28;41(1):91. doi: 10.1007/s10565-025-10038-2. Cell Biol Toxicol. 2025. PMID: 40437145 Free PMC article.
-
DTL promotes the growth and migration of melanoma cells through the ERK/E2F1/BUB1 axis.Sci Rep. 2024 Nov 1;14(1):26288. doi: 10.1038/s41598-024-76477-9. Sci Rep. 2024. PMID: 39487277 Free PMC article.
-
Identification of key genes and pathways in adrenocortical carcinoma: evidence from bioinformatic analysis.Front Endocrinol (Lausanne). 2023 Nov 20;14:1250033. doi: 10.3389/fendo.2023.1250033. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38053725 Free PMC article.
-
Inflammation in Urological Malignancies: The Silent Killer.Int J Mol Sci. 2023 Jan 3;24(1):866. doi: 10.3390/ijms24010866. Int J Mol Sci. 2023. PMID: 36614308 Free PMC article. Review.
-
Lipedema: Insights into Morphology, Pathophysiology, and Challenges.Biomedicines. 2022 Nov 30;10(12):3081. doi: 10.3390/biomedicines10123081. Biomedicines. 2022. PMID: 36551837 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. - PubMed
-
- von der Maase H, Sengelov L, Roberts JT, Ricci S, Dogliotti L, Oliver T, Moore MJ, Zimmermann A, Arning M. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23:4602–4608. - PubMed
-
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. - PubMed
-
- Liu H, Jia L, Yu H. Phospho-H2A and cohesin specify distinct tension-regulated Sgo1 pools at kinetochores and inner centromeres. Curr Biol. 2013;23:1927–1933. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

