Baseline [18F]GTP1 tau PET imaging is associated with subsequent cognitive decline in Alzheimer's disease
- PMID: 34852837
- PMCID: PMC8638526
- DOI: 10.1186/s13195-021-00937-x
Baseline [18F]GTP1 tau PET imaging is associated with subsequent cognitive decline in Alzheimer's disease
Abstract
Background: The role and implementation of tau PET imaging for predicting subsequent cognitive decline in Alzheimer's disease (AD) remains uncertain. This study was designed to evaluate the relationship between baseline [18F]GTP1 tau PET and subsequent longitudinal change across multiple cognitive measures over 18 months.
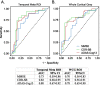
Methods: Our analyses incorporated data from 67 participants, including cognitively normal controls (n = 10) and β-amyloid (Aβ)-positive individuals ([18F] florbetapir Aβ PET) with prodromal (n = 26), mild (n = 16), or moderate (n = 15) AD. Baseline measurements included cortical volume (MRI), tau burden ([18F]GTP1 tau PET), and cognitive assessments [Mini-Mental State Examination (MMSE), Clinical Dementia Rating (CDR), 13-item version of the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog13), and Repeatable Battery for the Assessment of Neuropsychological Status (RBANS)]. Cognitive assessments were repeated at 6-month intervals over an 18-month period. Associations between baseline [18F]GTP1 tau PET indices and longitudinal cognitive performance were assessed via univariate (Spearman correlations) and multivariate (linear mixed effects models) approaches. The utility of potential prognostic tau PET cut points was assessed with ROC curves.
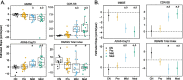
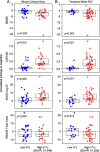
Results: Univariate analyses indicated that greater baseline [18F]GTP1 tau PET signal was associated with faster rates of subsequent decline on the MMSE, CDR, and ADAS-Cog13 across regions of interest (ROIs). In multivariate analyses adjusted for baseline age, cognitive performance, cortical volume, and Aβ PET SUVR, the prognostic performance of [18F]GTP1 SUVR was most robust in the whole cortical gray ROI. When AD participants were dichotomized into low versus high tau subgroups based on baseline [18F]GTP1 PET standardized uptake value ratios (SUVR) in the temporal (cutoff = 1.325) or whole cortical gray (cutoff = 1.245) ROIs, high tau subgroups demonstrated significantly more decline on the MMSE, CDR, and ADAS-Cog13.
Conclusions: Our results suggest that [18F]GTP1 tau PET represents a prognostic biomarker in AD and are consistent with data from other tau PET tracers. Tau PET imaging may have utility for identifying AD patients at risk for more rapid cognitive decline and for stratification and/or enrichment of participant selection in AD clinical trials. Trial registration ClinicalTrials.gov NCT02640092 . Registered on December 28, 2015.
Keywords: Alzheimer’s disease; Cognition; PET; Prognosis; Tau.
© 2021. The Author(s).
Conflict of interest statement
All authors are current or former employees or contractors of Genentech, Inc. and/or F. Hoffmann-La Roche, Ltd. All authors except SLB are current or former shareholders of F. Hoffmann La Roche, Ltd.
Figures


Similar articles
-
Cross-sectional and prognostic associations of baseline [18F]GTP1 tau PET signal and white matter lesion volumes for cognitive and functional decline in prodromal-to-mild Alzheimer's disease.J Alzheimers Dis. 2025 Jan;103(2):465-475. doi: 10.1177/13872877241302497. Epub 2025 Jan 12. J Alzheimers Dis. 2025. PMID: 39801050
-
Cross-sectional associations between [18F]GTP1 tau PET and cognition in Alzheimer's disease.Neurobiol Aging. 2019 Sep;81:138-145. doi: 10.1016/j.neurobiolaging.2019.05.026. Epub 2019 Jun 6. Neurobiol Aging. 2019. PMID: 31280117
-
Comparison of CSF markers and semi-quantitative amyloid PET in Alzheimer's disease diagnosis and in cognitive impairment prognosis using the ADNI-2 database.Alzheimers Res Ther. 2017 Apr 26;9(1):32. doi: 10.1186/s13195-017-0260-z. Alzheimers Res Ther. 2017. PMID: 28441967 Free PMC article.
-
What's the cut-point?: a systematic investigation of tau PET thresholding methods.Alzheimers Res Ther. 2022 Apr 5;14(1):49. doi: 10.1186/s13195-022-00986-w. Alzheimers Res Ther. 2022. PMID: 35382866 Free PMC article. Review.
-
Minimal clinically important difference in Alzheimer's disease: Rapid review.Alzheimers Dement. 2024 May;20(5):3352-3363. doi: 10.1002/alz.13770. Epub 2024 Apr 1. Alzheimers Dement. 2024. PMID: 38561021 Free PMC article.
Cited by
-
Explainable Artificial Intelligence in Neuroimaging of Alzheimer's Disease.Diagnostics (Basel). 2025 Mar 4;15(5):612. doi: 10.3390/diagnostics15050612. Diagnostics (Basel). 2025. PMID: 40075859 Free PMC article. Review.
-
Default mode network tau predicts future clinical decline in atypical early Alzheimer's disease.Brain. 2025 Apr 3;148(4):1329-1344. doi: 10.1093/brain/awae327. Brain. 2025. PMID: 39412999
-
Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline.Nat Med. 2022 Nov;28(11):2381-2387. doi: 10.1038/s41591-022-02049-x. Epub 2022 Nov 10. Nat Med. 2022. PMID: 36357681 Free PMC article.
-
Evaluation of partial volume correction and analysis of longitudinal [18F]GTP1 tau PET imaging in Alzheimer's disease using linear mixed-effects models.Front Neuroimaging. 2024 Mar 28;3:1355402. doi: 10.3389/fnimg.2024.1355402. eCollection 2024. Front Neuroimaging. 2024. PMID: 38606196 Free PMC article.
-
A simple genetic stratification method for lower cost, more expedient clinical trials in early Alzheimer's disease: A preliminary study of tau PET and cognitive outcomes.Alzheimers Dement. 2023 Jul;19(7):3078-3086. doi: 10.1002/alz.12952. Epub 2023 Jan 26. Alzheimers Dement. 2023. PMID: 36701211 Free PMC article.
References
-
- Querfurth HW, LaFerla FM. Alzheimer’s disease. N Engl J Med. 2010;362:329–344. - PubMed
-
- Sanabria Bohorquez S, Marik J, Ogasawara A, Tinianow JN, Gill HS, Barret O, et al. [(18)F]GTP1 (Genentech Tau Probe 1), a radioligand for detecting neurofibrillary tangle tau pathology in Alzheimer’s disease. Eur J Nucl Med Mol Imaging. 2019;46:2077–2089. - PubMed
-
- Lohith TG, Bennacef I, Vandenberghe R, Vandenbulcke M, Salinas CA, Declercq R, et al. Brain imaging of Alzheimer dementia patients and elderly controls with (18)F-MK-6240, a PET tracer targeting neurofibrillary tangles. J Nucl Med. 2019;60:107–114. - PubMed

