Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial
- PMID: 34853024
- PMCID: PMC8634365
- DOI: 10.1136/bmj-2021-065217
Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial
Erratum in
-
Financial incentives for smoking cessation in pregnancy: multicentre randomised controlled trial.BMJ. 2021 Dec 3;375:n3012. doi: 10.1136/bmj.n3012. BMJ. 2021. PMID: 34862197 Free PMC article. No abstract available.
-
Correction for vol. 376, p.BMJ. 2022 Feb 22;376:o448. doi: 10.1136/bmj.o448. BMJ. 2022. PMID: 35193871 Free PMC article.
Abstract
Objective: To evaluate the efficacy of financial incentives dependent on continuous smoking abstinence on smoking cessation and birth outcomes among pregnant smokers.
Design: Single blind, randomised controlled trial.
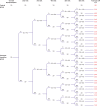
Setting: Financial Incentive for Smoking Cessation in Pregnancy (FISCP) trial in 18 maternity wards in France.
Participants: 460 pregnant smokers aged at least 18 years who smoked ≤5 cigarettes/day or ≤3 roll-your-own cigarettes/day and had a pregnancy gestation of <18 weeks were randomised to a financial incentives group (n=231) or a control group (n=229).
Interventions: Participants in the financial incentives group received a voucher equivalent to €20 (£17; $23), and further progressively increasing vouchers at each study visit if they remained abstinent. Participants in the control group received no financial incentive for abstinence. All participants received a €20 show-up fee at each of six visits.
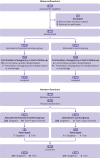
Main outcome measures: The main outcome measure was continuous smoking abstinence from the first post-quit date visit to visit 6, before delivery. Secondary outcomes in the mothers were point prevalence abstinence, time to smoking relapse, withdrawal symptoms, blood pressure, and alcohol and cannabis use in past 30 days. Secondary outcomes in the babies were gestational age at birth, birth characteristics (birth weight, length, head circumference, Apgar score), and a poor neonatal outcome-a composite measure of transfer to the neonatal unit, congenital malformation, convulsions, or perinatal death.
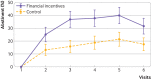
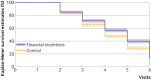
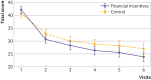
Results: Mean age was 29 years. In the financial incentives and control groups, respectively, 137 (59%) and 148 (65%) were employed, 163 (71%) and 171 (75%) were in a relationship, and 41 (18%) and 31 (13%) were married. The participants had smoked a median of 60 cigarettes in the past seven days. The continuous abstinence rate was significantly higher in the financial incentives group (16%, 38/231) than control group (7%, 17/229): odds ratio 2.45 (95% confidence interval 1.34 to 4.49), P=0.004). The point prevalence abstinence rate was higher (4.61, 1.41 to 15.01, P=0.011), the median time to relapse was longer (visit 5 (interquartile range 3-6) and visit 4 (3-6), P<0.001)), and craving for tobacco was lower (β=-1.81, 95% confidence interval -3.55 to -0.08, P=0.04) in the financial incentives group than control group. Financial incentives were associated with a 7% reduction in the risk of a poor neonatal outcome: 4 babies (2%) in the financial incentives group and 18 babies (9%) in the control group: mean difference 14 (95% confidence interval 5 to 23), P=0.003. Post hoc analyses suggested that more babies in the financial incentives group had birth weights ≥2500 g than in the control group: unadjusted odds ratio 1.95 (95% confidence interval 0.99 to 3.85), P=0.055; sex adjusted odds ratio 2.05 (1.03 to 4.10), P=0.041; and sex and prematurity adjusted odds ratio 2.06 (0.90 to 4.71), P=0.086. As these are post hoc analyses, the results should be interpreted with caution.
Conclusions: Financial incentives to reward smoking abstinence compared with no financial incentives were associated with an increased abstinence rate in pregnant smokers. Financial incentives dependent on smoking abstinence could be implemented as a safe and effective intervention to help pregnant smokers quit smoking.
Trial registration: ClinicalTrials.gov NCT02606227.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: no support from the French National Cancer Institute (INCa); no support from any organisation for the submitted work with the exception of LG who was supported by the study’s grant and by a grant from the health chair—a joint initiative between Paris Sciences et Lettres (PSL), Université Paris-Dauphine, l’École nationale de la statistique et de l’administration économique (ENSAE), Mutuelle générale de l’Éducation nationale (MGEN), Université Paris-Dauphine, and ISTYA Collectives under the aegis of the Fondation du Risque as a postdoctoral fellow. All authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
Comment in
-
Vouchers as an incentive to stop smoking.Br Dent J. 2022 Jan;232(1):37. doi: 10.1038/s41415-022-3843-4. Br Dent J. 2022. PMID: 35031742 No abstract available.
-
Financial incentives for health should be standard practice.BMJ. 2022 Jan 24;376:o179. doi: 10.1136/bmj.o179. BMJ. 2022. PMID: 35074862 No abstract available.
References
-
- National Centre for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health . The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Centers for Disease Control and Prevention, 2014. - PubMed
-
- US Centres for Disease Control and Prevention, U.S. Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. CDC, 2006. www.ncbi.nlm.nih.gov/books/NBK44324/
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
