Hypocretin/Orexin Interactions with Norepinephrine Contribute to the Opiate Withdrawal Syndrome
- PMID: 34853083
- PMCID: PMC8802943
- DOI: 10.1523/JNEUROSCI.1557-21.2021
Hypocretin/Orexin Interactions with Norepinephrine Contribute to the Opiate Withdrawal Syndrome
Abstract
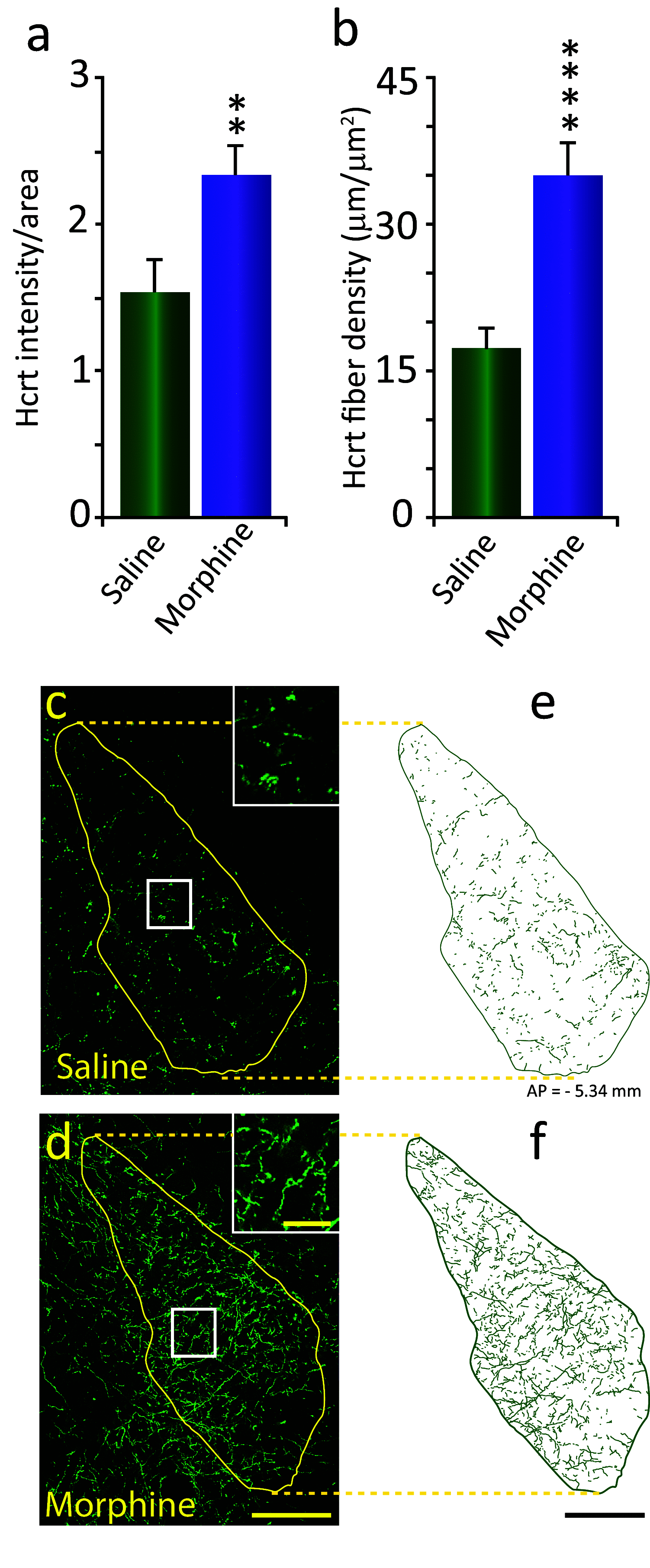
We previously found that human heroin addicts and mice chronically exposed to morphine exhibit a significant increase in the number of detected hypocretin/orexin (Hcrt)-producing neurons. However, it remains unknown how this increase affects target areas of the hypocretin system involved in opioid withdrawal, including norepinephrine containing structures locus coeruleus (LC) and A1/A2 medullary regions. Using a combination of immunohistochemical, biochemical, imaging, and behavioral techniques, we now show that the increase in detected hypocretin cell number translates into a significant increase in hypocretin innervation and tyrosine hydroxylase (TH) levels in the LC without affecting norepinephrine-containing neuronal cell number. We show that the increase in TH is completely dependent on Hcrt innervation. The A1/A2 regions were unaffected by morphine treatment. Manipulation of the Hcrt system may affect opioid addiction and withdrawal.SIGNIFICANCE STATEMENT Previously, we have shown that the hypothalamic hypocretin system undergoes profound anatomic changes in human heroin addicts and in mice exposed to morphine, suggesting a role of this system in the development of addictive behaviors. The locus coeruleus plays a key role in opioid addiction. Here we report that the hypothalamic hypocretin innervation of the locus coeruleus increases dramatically with morphine administration to mice. This increase is correlated with a massive increase in tyrosine hydroxylase expression in locus coeruleus. Elimination of hypocretin neurons prevents the tyrosine hydroxylase increase in locus coeruleus and dampens the somatic and affective components of opioid withdrawal.
Keywords: addiction; anatomy; hypocretin; locus coeruleus; opioids; withdrawal.
Copyright © 2022 the authors.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
