Respiration, Heartbeat, and Conscious Tactile Perception
- PMID: 34853084
- PMCID: PMC8805629
- DOI: 10.1523/JNEUROSCI.0592-21.2021
Respiration, Heartbeat, and Conscious Tactile Perception
Abstract
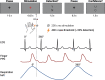
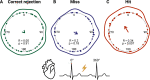
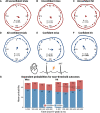
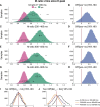
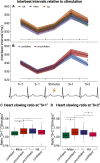
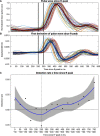
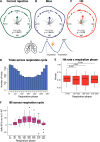
Previous studies have shown that timing of sensory stimulation during the cardiac cycle interacts with perception. Given the natural coupling of respiration and cardiac activity, we investigated here their joint effects on tactile perception. Forty-one healthy female and male human participants reported conscious perception of finger near-threshold electrical pulses (33% null trials) and decision confidence while electrocardiography, respiratory activity, and finger photoplethysmography were recorded. Participants adapted their respiratory cycle to expected stimulus onsets to preferentially occur during late inspiration/early expiration. This closely matched heart rate variation (sinus arrhythmia) across the respiratory cycle such that most frequent stimulation onsets occurred during the period of highest heart rate probably indicating highest alertness and cortical excitability. Tactile detection rate was highest during the first quadrant after expiration onset. Interindividually, stronger respiratory phase-locking to the task was associated with higher detection rates. Regarding the cardiac cycle, we confirmed previous findings that tactile detection rate was higher during diastole than systole and newly specified its minimum at 250-300 ms after the R-peak corresponding to the pulse wave arrival in the finger. Expectation of stimulation induced a transient heart deceleration which was more pronounced for unconfident decision ratings. Interindividually, stronger poststimulus modulations of heart rate were linked to higher detection rates. In summary, we demonstrate how tuning to the respiratory cycle and integration of respiratory-cardiac signals are used to optimize performance of a tactile detection task.SIGNIFICANCE STATEMENT Mechanistic studies on perception and cognition tend to focus on the brain neglecting contributions of the body. Here, we investigated how respiration and heartbeat influence tactile perception: respiration phase-locking to expected stimulus onsets corresponds to highest heart rate (and presumably alertness/cortical excitability) and correlates with detection performance. Tactile detection varies across the heart cycle with a minimum when the pulse reaches the finger and a maximum in diastole. Taken together with our previous finding of unchanged early event-related potentials across the cardiac cycle, we conclude that these effects are not a peripheral physiological artifact but a result of cognitive processes that model our body's internal state, make predictions to guide behavior, and might also tune respiration to serve the task.
Keywords: cardiac cycle; electrocardiogram; interoception; photoplethysmography; respiration; tactile perception.
Copyright © 2022 the authors.
Figures
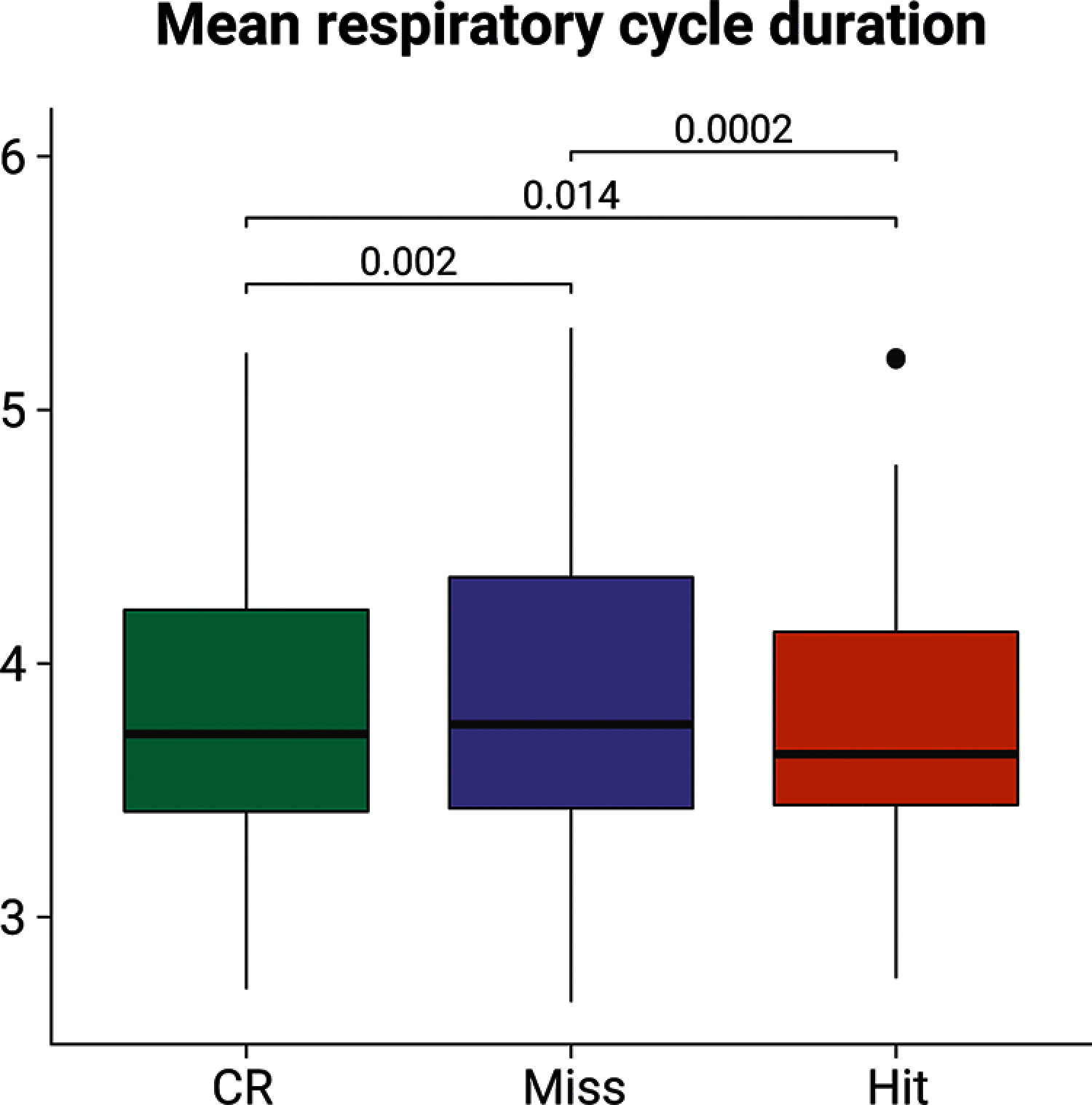
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
