Switch receptor T3/28 improves long-term persistence and antitumor efficacy of CAR-T cells
- PMID: 34853180
- PMCID: PMC8638458
- DOI: 10.1136/jitc-2021-003176
Switch receptor T3/28 improves long-term persistence and antitumor efficacy of CAR-T cells
Abstract
Background: Chimeric antigen receptor (CAR) T cells have been successfully used in tumor immunotherapy due to their strong antitumor responses, especially in hematological malignancies such as B cell acute lymphoid leukemia. However, on-target off-tumor toxicity and poor persistence severely limit the clinical application of CAR-T cell therapy.
Methods: T-cell immunoglobulin mucin domain molecule 3 (TIM-3) was used to develop a second-generation 41BB CD19 CAR linked with a T3/28 chimera, in which truncated extracellular TIM-3 was fused with the CD28 transmembrane and cytoplasmic domains. The efficacy of T3/28 CAR-T cells was evaluated in vitro and in vivo.
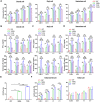
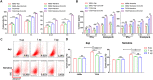
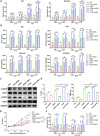
Results: We demonstrated that the switch receptor T3/28 preserved the TCM phenotype, improved proliferative capacity, and reduced exhaustion of CAR-T cells, resulting in superior in vitro and in vivo antitumor activity in B lymphoma. Importantly, the switch receptor T3/28 substantially prolonged the persistence of CAR-T cells, and the interleukin-21/Stat3 axis probably contributed to the enhanced cytotoxicity of T3/28 CAR-T cells.
Conclusion: Overall, the T3/28 chimera significantly prolonged the persistence of CAR-T cells, and T3/28 CAR-T cells possessed potent antitumor activity in mice, shedding new light on potential improvements in adoptive T cell therapies.
Keywords: immunity; immunotherapy.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures




Similar articles
-
Tethered IL15-IL15Rα augments antitumor activity of CD19 CAR-T cells but displays long-term toxicity in an immunocompetent lymphoma mouse model.J Immunother Cancer. 2024 Jul 1;12(7):e008572. doi: 10.1136/jitc-2023-008572. J Immunother Cancer. 2024. PMID: 38955421 Free PMC article.
-
NR4A ablation improves mitochondrial fitness for long persistence in human CAR-T cells against solid tumors.J Immunother Cancer. 2024 Aug 16;12(8):e008665. doi: 10.1136/jitc-2023-008665. J Immunother Cancer. 2024. PMID: 39151930 Free PMC article.
-
NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma.Clin Cancer Res. 2019 Dec 1;25(23):7126-7138. doi: 10.1158/1078-0432.CCR-19-0421. Epub 2019 Sep 4. Clin Cancer Res. 2019. PMID: 31484667 Free PMC article.
-
Prolonged Persistence of Chimeric Antigen Receptor (CAR) T Cell in Adoptive Cancer Immunotherapy: Challenges and Ways Forward.Front Immunol. 2020 Apr 22;11:702. doi: 10.3389/fimmu.2020.00702. eCollection 2020. Front Immunol. 2020. PMID: 32391013 Free PMC article. Review.
-
Chimeric Antigen Receptor T Cell Therapy for Pediatric B-ALL: Narrowing the Gap Between Early and Long-Term Outcomes.Front Immunol. 2020 Aug 11;11:1985. doi: 10.3389/fimmu.2020.01985. eCollection 2020. Front Immunol. 2020. PMID: 32849662 Free PMC article. Review.
Cited by
-
Design and Evaluation of TIM-3-CD28 Checkpoint Fusion Proteins to Improve Anti-CD19 CAR T-Cell Function.Front Immunol. 2022 Apr 6;13:845499. doi: 10.3389/fimmu.2022.845499. eCollection 2022. Front Immunol. 2022. PMID: 35464394 Free PMC article.
-
Targeting LAG-3, TIM-3, and TIGIT for cancer immunotherapy.J Hematol Oncol. 2023 Sep 5;16(1):101. doi: 10.1186/s13045-023-01499-1. J Hematol Oncol. 2023. PMID: 37670328 Free PMC article. Review.
-
Harnessing the tumor microenvironment to boost adoptive T cell therapy with engineered lymphocytes for solid tumors.Semin Immunopathol. 2024 Jul 25;46(3-4):8. doi: 10.1007/s00281-024-01011-y. Semin Immunopathol. 2024. PMID: 39060547 Review.
-
Focusing on CD8+ T-cell phenotypes: improving solid tumor therapy.J Exp Clin Cancer Res. 2024 Sep 28;43(1):266. doi: 10.1186/s13046-024-03195-5. J Exp Clin Cancer Res. 2024. PMID: 39342365 Free PMC article. Review.
-
Modular pooled discovery of synthetic knockin sequences to program durable cell therapies.Cell. 2023 Sep 14;186(19):4216-4234.e33. doi: 10.1016/j.cell.2023.08.013. Cell. 2023. PMID: 37714135 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
