Counteraction between Astrin-PP1 and Cyclin-B-CDK1 pathways protects chromosome-microtubule attachments independent of biorientation
- PMID: 34853300
- PMCID: PMC8636589
- DOI: 10.1038/s41467-021-27131-9
Counteraction between Astrin-PP1 and Cyclin-B-CDK1 pathways protects chromosome-microtubule attachments independent of biorientation
Abstract
Defects in chromosome-microtubule attachment can cause chromosomal instability (CIN), frequently associated with infertility and aggressive cancers. Chromosome-microtubule attachment is mediated by a large macromolecular structure, the kinetochore. Sister kinetochores of each chromosome are pulled by microtubules from opposing spindle-poles, a state called biorientation which prevents chromosome missegregation. Kinetochore-microtubule attachments that lack the opposing-pull are detached by Aurora-B/Ipl1. It is unclear how mono-oriented attachments that precede biorientation are spared despite the lack of opposing-pull. Using an RNAi-screen, we uncover a unique role for the Astrin-SKAP complex in protecting mono-oriented attachments. We provide evidence of domains in the microtubule-end associated protein that sense changes specific to end-on kinetochore-microtubule attachments and assemble an outer-kinetochore crescent to stabilise attachments. We find that Astrin-PP1 and Cyclin-B-CDK1 pathways counteract each other to preserve mono-oriented attachments. Thus, CIN prevention pathways are not only surveying attachment defects but also actively recognising and stabilising mature attachments independent of biorientation.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
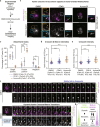

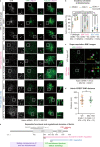
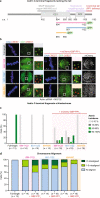
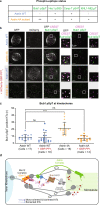

Similar articles
-
Kinetochores attached to microtubule-ends are stabilised by Astrin bound PP1 to ensure proper chromosome segregation.Elife. 2019 Dec 6;8:e49325. doi: 10.7554/eLife.49325. Elife. 2019. PMID: 31808746 Free PMC article.
-
The Astrin-SKAP complex reduces friction at the kinetochore-microtubule interface.Curr Biol. 2022 Jun 20;32(12):2621-2631.e3. doi: 10.1016/j.cub.2022.04.061. Epub 2022 May 16. Curr Biol. 2022. PMID: 35580605 Free PMC article.
-
The association of Plk1 with the astrin-kinastrin complex promotes formation and maintenance of a metaphase plate.J Cell Sci. 2021 Jan 8;134(1):jcs251025. doi: 10.1242/jcs.251025. J Cell Sci. 2021. PMID: 33288550 Free PMC article.
-
Regulation of kinetochore-microtubule attachments by Aurora B kinase.Biochem Soc Trans. 2009 Oct;37(Pt 5):976-80. doi: 10.1042/BST0370976. Biochem Soc Trans. 2009. PMID: 19754435 Review.
-
Orchestration of the spindle assembly checkpoint by CDK1-cyclin B1.FEBS Lett. 2019 Oct;593(20):2889-2907. doi: 10.1002/1873-3468.13591. Epub 2019 Sep 13. FEBS Lett. 2019. PMID: 31469407 Review.
Cited by
-
KIF18A and CDK1 as combined therapeutic targets in cervical and endometrial carcinomas: based on bioinformatics analysis and in vitro experiments.Transl Cancer Res. 2024 Dec 31;13(12):6880-6894. doi: 10.21037/tcr-24-1831. Epub 2024 Dec 18. Transl Cancer Res. 2024. PMID: 39816554 Free PMC article.
-
Search for chromosomal instability aiding variants reveal naturally occurring kinetochore gene variants that perturb chromosome segregation.iScience. 2024 Jan 26;27(3):109007. doi: 10.1016/j.isci.2024.109007. eCollection 2024 Mar 15. iScience. 2024. PMID: 38361632 Free PMC article.
-
CCHCR1-astrin interaction promotes centriole duplication through recruitment of CEP72.BMC Biol. 2022 Oct 24;20(1):240. doi: 10.1186/s12915-022-01437-6. BMC Biol. 2022. PMID: 36280838 Free PMC article.
-
Polar Chromosomes-Challenges of a Risky Path.Cells. 2022 May 3;11(9):1531. doi: 10.3390/cells11091531. Cells. 2022. PMID: 35563837 Free PMC article. Review.
-
Liquid-liquid phase separation of microtubule-binding proteins in the regulation of spindle assembly.Cell Prolif. 2024 Oct;57(10):e13649. doi: 10.1111/cpr.13649. Epub 2024 May 13. Cell Prolif. 2024. PMID: 38736355 Free PMC article. Review.
References
-
- Roos U-P. Light and electron microscopy of rat kangaroo cells in mitosis. Chromosoma. 1976;54:363–385. - PubMed
-
- Cassimeris L, Rieder CL, Salmon ED. Microtubule assembly and kinetochore directional instability in vertebrate monopolar spindles: implications for the mechanism of chromosome congression. J. Cell. Sci. 1994;107:285–297. - PubMed
-
- Ben-David U, Amon A. Context is everything: aneuploidy in cancer. Nat. Rev. Genet. 2020;21:44–62. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01003X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/T017716/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/W002698/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- C28598/A9787/CRUK_/Cancer Research UK/United Kingdom
- MR/K50127X/1 /MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

