Performance and usefulness of a novel automated immunoassay HISCL SARS-CoV-2 Antigen assay kit for the diagnosis of COVID-19
- PMID: 34853366
- PMCID: PMC8636628
- DOI: 10.1038/s41598-021-02636-x
Performance and usefulness of a novel automated immunoassay HISCL SARS-CoV-2 Antigen assay kit for the diagnosis of COVID-19
Erratum in
-
Author Correction: Performance and usefulness of a novel automated immunoassay HISCL SARS-CoV-2 Antigen assay kit for the diagnosis of COVID-19.Sci Rep. 2021 Dec 8;11(1):23968. doi: 10.1038/s41598-021-03518-y. Sci Rep. 2021. PMID: 34880380 Free PMC article. No abstract available.
Abstract
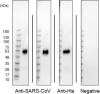
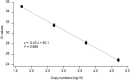
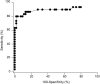
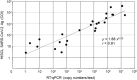
Here, we aimed to evaluate the clinical performance of a novel automated immunoassay HISCL SARS-CoV-2 Antigen assay kit designed to detect the nucleocapsid (N) protein of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). This kit comprises automated chemiluminescence detection systems. Western blot analysis confirmed that anti-SARS-CoV antibodies detected SARS-CoV-2N proteins. The best cut-off index was determined, and clinical performance was tested using 115 serum samples obtained from 46 patients with coronavirus disease 2019 (COVID-19) and 69 individuals who tested negative for COVID-19 through reverse transcription quantitative polymerase chain reaction (RT-qPCR). The HISCL Antigen assay kit showed a sensitivity of 95.4% and 16.6% in samples with copy numbers > 100 and < 99, respectively. The kit did not cross-react with human coronaviruses causing seasonal common cold and influenza, and none of the 69 individuals without COVID-19 were diagnosed with positive results. Importantly, 81.8% of the samples with low virus load (< 50 copy numbers) were diagnosed as negative. Thus, using HISCL antigen assay kits may reduce overdiagnosis compared with RT-qPCR tests. The rapid and high-throughput HISCL SARS-CoV-2 Antigen assay kit developed here proved suitable for screening infectious COVID-19 and may help control the pandemic.
© 2021. The Author(s).
Conflict of interest statement
Sysmex Corporation provided reagents for HISCL SARS-CoV-2 Antigen assay kit measurements free of charge. Akinori Kawai, Jun Matsui, Yoshiyuki Fukushima, Norihiro Kikukawa, and Takuya Kyoutou are employees of Sysmex Corporation. The other authors (Kaori Saito, Tomohiko Ai, Masayoshi Chonan, Takeaki Kawakami, Yoshie Hosaka, Shigeki Misawa, Haruhi Takagi, Yasushi Matsushita, Makoto Hiki, Atsushi Okuzawa, Satoshi Hori, Toshio Naito, Takashi Miida, Kazuhisa Takahashi, and Yoko Tabe) do not have COI to disclose. This study was performed following strict adherence to academic standards.
Figures
References
-
- Zdravkovic M, Berger-Estilita J, Zdravkovic B, Berger D. Scientific quality of COVID-19 and SARS CoV-2 publications in the highest impact medical journals during the early phase of the pandemic: A case control study. PLoS ONE. 2020;15:e0241826. doi: 10.1371/journal.pone.0241826. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

