Gut and Cutaneous Microbiome Featuring Abundance of Lactobacillus reuteri Protected Against Psoriasis-Like Inflammation in Mice
- PMID: 34853526
- PMCID: PMC8627893
- DOI: 10.2147/JIR.S337031
Gut and Cutaneous Microbiome Featuring Abundance of Lactobacillus reuteri Protected Against Psoriasis-Like Inflammation in Mice
Abstract
Background: Psoriasis is a chronic autoinflammatory skin disease, and its aetiology remains incompletely understood. Recently, gut microbial dysbiosis is found to be tightly associated with psoriasis.
Objective: We sought to reveal the causal role of gut microbiota dysbiosis in psoriasis pathogenesis and investigate the protective effect of healthy commensal bacteria against imiquimod -induced psoriasis-like skin response.
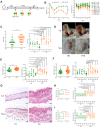
Methods: By using fecal microbial transplantation (FMT), 16S rRNA gene-based taxonomic profiling and Lactobacillus supplement, we have assessed the effect of FMT from healthy individuals on psoriasis-like skin inflammation and associated immune disorders in imiquimod-induced psoriasis mice.
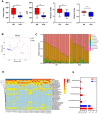
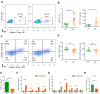
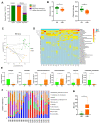
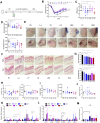
Results: Here, by using psoriasis mice humanized with the stools from healthy donors and psoriasis patients, the imiquimod-induced psoriasis in mice with psoriasis patient stool was found to be significantly aggravated as compared to the mice with healthy donor stools. Further analysis showed fecal microbiota of healthy individuals protected against Treg/Th17 imbalance in psoriasis. Moreover, we found the gut and skin microbiome in mice receipted with gut microbiota of healthy individuals (HD) differed from those of mice receipted with gut microbiota of psoriasis patients (PSD). 16S rRNA sequencing revealed that Lactobacillus reuteri was greatly enriched in fecal and cutaneous microbiome of HD mice as compared to PSD mice. Intriguingly, supplement with Lactobacillus reuteri was sufficient to increase the expression of anti-inflammatory gene IL-10, reduce Th17 cells counts and confer resistance to imiquimod-induced inflammation on the mice with gut microbiota dysbiosis.
Conclusion: Our results suggested that the gut microbiota dysbiosis is the potential causal factor for psoriasis and the gut microbiota may serve as promising therapy target for psoriasis patients.
Keywords: Lactobacillus reuteri; Th17; cutaneous microbiome; fecal microbiota transplantation; gut microbiota; psoriasis.
© 2021 Chen et al.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources

