Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma: A systematic review
- PMID: 34853653
- PMCID: PMC8603457
- DOI: 10.4251/wjgo.v13.i11.1813
Atezolizumab plus bevacizumab versus sorafenib or atezolizumab alone for unresectable hepatocellular carcinoma: A systematic review
Abstract
Background: Despite the use of current standard therapy, the prognosis of patients with unresectable hepatocellular carcinoma (HCC) is poor, with median survival times of 40 mo for intermediate HCC (Barcelona Clinic Liver Cancer [BCLC] stage B) and 6-8 mo for advanced HCC (BCLC stage C). Although patients with early-stage HCC are usually suitable for therapies with curative intention, up to 70% of patients experience relapse within 5 years. In the past decade, the United States Food and Drug Administration has approved different immunogenic treatment options for advanced HCC, the most common type of liver cancer among adults. Nevertheless, no treatment is useful in the adjuvant setting. Since 2007, the multi-kinase inhibitor sorafenib has been used as a first-line targeted drug to address the increased mortality and incidence rates of HCC. However, in 2020, the IMbrave150 trial demonstrated that combination therapy of atezolizumab (anti-programmed death-ligand 1 [PD-L1]) and bevacizumab (anti-vascular endothelial growth factor [VEGF]) is superior to sorafenib, a single anti-programmed death 1/PD-L1 antibody inhibitor used as an anti-cancer monotherapy for HCC treatment.
Aim: To conduct a systematic literature review to evaluate the evidence supporting the efficacy and safety of atezolizumab/bevacizumab as preferred first-line drug therapy over the conventional sorafenib or atezolizumab monotherapies, which are used to improve survival outcomes and reduce disease progression in patients with unresectable HCC and non-decompensated liver disease.
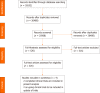
Methods: A comprehensive literature review was conducted using the PubMed, Scopus, ScienceDirect, clinicaltrials.gov, PubMed Central, Embase, EuropePMC, and CINAHL databases to identify studies that met the inclusion criteria using relevant MeSH terms. This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines and risk of bias (RoB) were assessed using the Cochrane RoB 2 tool and Sevis.
Results: In the atezolizumab/bevacizumab group, an improvement in overall tumor response, reduction of disease progression, and longer progression-free survival were observed compared to monotherapy with either sorafenib or atezolizumab. Hypertension and proteinuria were the most common adverse events, and the rates of adverse events were comparable to those with the monotherapy. Of the studies, there were two completed trials and two ongoing trials analyzed using high quality and low bias. A more thorough analysis was only performed on the completed trials.
Conclusion: Treatment of HCC with atezolizumab/bevacizumab combination therapy was confirmed to be an effective first-line treatment to improve survival in patients with unresectable HCC and non-decompensated liver disease compared to monotherapy with either sorafenib or atezolizumab.
Keywords: Barcelona clinic liver cancer; Combination systemic therapy; Hepatic malignancy; Immunogenetic therapy; Liver transplantation; Transarterial chemoembolization.
©The Author(s) 2021. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interests for this article.
Figures
Similar articles
-
Atezolizumab with or without bevacizumab in unresectable hepatocellular carcinoma (GO30140): an open-label, multicentre, phase 1b study.Lancet Oncol. 2020 Jun;21(6):808-820. doi: 10.1016/S1470-2045(20)30156-X. Lancet Oncol. 2020. PMID: 32502443 Clinical Trial.
-
IMbrave150: Efficacy and Safety of Atezolizumab plus Bevacizumab versus Sorafenib in Patients with Barcelona Clinic Liver Cancer Stage B Unresectable Hepatocellular Carcinoma: An Exploratory Analysis of the Phase III Study.Liver Cancer. 2022 Nov 28;12(3):238-250. doi: 10.1159/000528272. eCollection 2023 Aug. Liver Cancer. 2022. PMID: 37767068 Free PMC article.
-
Patient-reported outcomes with atezolizumab plus bevacizumab versus sorafenib in patients with unresectable hepatocellular carcinoma (IMbrave150): an open-label, randomised, phase 3 trial.Lancet Oncol. 2021 Jul;22(7):991-1001. doi: 10.1016/S1470-2045(21)00151-0. Epub 2021 May 27. Lancet Oncol. 2021. PMID: 34051880 Clinical Trial.
-
Transarterial chemoembolization combined with atezolizumab plus bevacizumab conversion therapy for intermediate-stage hepatocellular carcinoma: a case report and literature review.Front Immunol. 2024 May 28;15:1358602. doi: 10.3389/fimmu.2024.1358602. eCollection 2024. Front Immunol. 2024. PMID: 38863699 Free PMC article. Review.
-
First-Line Targeted Therapy for Hepatocellular Carcinoma: Role of Atezolizumab/Bevacizumab Combination.Biomedicines. 2022 Jun 2;10(6):1304. doi: 10.3390/biomedicines10061304. Biomedicines. 2022. PMID: 35740326 Free PMC article. Review.
Cited by
-
Efficacy and safety of HAIC alone vs. HAIC combined with lenvatinib for treatment of advanced hepatocellular carcinoma.Med Oncol. 2023 Apr 12;40(5):147. doi: 10.1007/s12032-023-02012-x. Med Oncol. 2023. PMID: 37043113
-
Differential in vitro effects of targeted therapeutics in primary human liver cancer: importance for combined liver cancer.BMC Cancer. 2022 Nov 19;22(1):1193. doi: 10.1186/s12885-022-10247-6. BMC Cancer. 2022. PMID: 36402986 Free PMC article.
-
Updating the Clinical Application of Blood Biomarkers and Their Algorithms in the Diagnosis and Surveillance of Hepatocellular Carcinoma: A Critical Review.Int J Mol Sci. 2023 Feb 21;24(5):4286. doi: 10.3390/ijms24054286. Int J Mol Sci. 2023. PMID: 36901717 Free PMC article. Review.
-
Microwave ablation as a primary versus secondary treatment for hepatocellular carcinoma.Diagn Interv Radiol. 2023 Mar 29;29(2):359-366. doi: 10.4274/dir.2023.221930. Epub 2023 Mar 1. Diagn Interv Radiol. 2023. PMID: 36988024 Free PMC article.
-
The GALAD score and the BALAD-2 score correlate with transarterial and systemic treatment response and survival in patients with hepatocellular carcinoma.J Cancer Res Clin Oncol. 2024 Feb 6;150(2):81. doi: 10.1007/s00432-023-05526-z. J Cancer Res Clin Oncol. 2024. PMID: 38319485 Free PMC article.
References
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. - PubMed
-
- Brown ZJ, Greten TF, Heinrich B. Adjuvant Treatment of Hepatocellular Carcinoma: Prospect of Immunotherapy. Hepatology. 2019;70:1437–1442. - PubMed
-
- Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, Cao Y, Shujath J, Gawlak S, Eveleigh D, Rowley B, Liu L, Adnane L, Lynch M, Auclair D, Taylor I, Gedrich R, Voznesensky A, Riedl B, Post LE, Bollag G, Trail PA. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous