Hepatitis B x antigen (HBx) is an important therapeutic target in the pathogenesis of hepatocellular carcinoma
- PMID: 34853663
- PMCID: PMC8629409
- DOI: 10.18632/oncotarget.28077
Hepatitis B x antigen (HBx) is an important therapeutic target in the pathogenesis of hepatocellular carcinoma
Abstract
Hepatitis B virus (HBV) is a human pathogen that has infected an estimated two billion people worldwide. Despite the availability of highly efficacious vaccines, universal screening of the blood supply for virus, and potent direct acting anti-viral drugs, there are more than 250 million carriers of HBV who are at risk for the sequential development of hepatitis, fibrosis, cirrhosis and hepatocellular carcinoma (HCC). More than 800,000 deaths per year are attributed to chronic hepatitis B. Many different therapeutic approaches have been developed to block virus replication, and although effective, none are curative. These treatments have little or no impact upon the portions of integrated HBV DNA, which often encode the virus regulatory protein, HBx. Although given little attention, HBx is an important therapeutic target because it contributes importantly to (a) HBV replication, (b) in protecting infected cells from immune mediated destruction during chronic infection, and (c) in the development of HCC. Thus, the development of therapies targeting HBx, combined with other established therapies, will provide a functional cure that will target virus replication and further reduce or eliminate both the morbidity and mortality associated with chronic liver disease and HCC. Simultaneous targeting of all these characteristics underscores the importance of developing therapies against HBx.
Keywords: HBx; chronic liver disease; epigenetic; functional cure; hepatocellular carcinoma.
Copyright: © 2021 Medhat et al.
Conflict of interest statement
CONFLICTS OF INTEREST Authors have no conflicts of interest to declare.
Figures
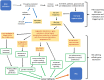
Similar articles
-
Hepatitis B x (HBx) as a Component of a Functional Cure for Chronic Hepatitis B.Biomedicines. 2022 Sep 7;10(9):2210. doi: 10.3390/biomedicines10092210. Biomedicines. 2022. PMID: 36140311 Free PMC article. Review.
-
The Epigenetic Modulation of Cancer and Immune Pathways in Hepatitis B Virus-Associated Hepatocellular Carcinoma: The Influence of HBx and miRNA Dysregulation.Front Immunol. 2021 Apr 29;12:661204. doi: 10.3389/fimmu.2021.661204. eCollection 2021. Front Immunol. 2021. PMID: 33995383 Free PMC article. Review.
-
The roles of hepatitis B virus-encoded X protein in virus replication and the pathogenesis of chronic liver disease.Expert Opin Ther Targets. 2014 Mar;18(3):293-306. doi: 10.1517/14728222.2014.867947. Epub 2014 Jan 3. Expert Opin Ther Targets. 2014. PMID: 24387282 Review.
-
The novel HBx mutation F30V correlates with hepatocellular carcinoma in vivo, reduces hepatitis B virus replicative efficiency and enhances anti-apoptotic activity of HBx N terminus in vitro.Clin Microbiol Infect. 2019 Jul;25(7):906.e1-906.e7. doi: 10.1016/j.cmi.2018.11.017. Epub 2018 Nov 23. Clin Microbiol Infect. 2019. PMID: 30472417
-
Host Epigenetic Alterations and Hepatitis B Virus-Associated Hepatocellular Carcinoma.J Clin Med. 2021 Apr 16;10(8):1715. doi: 10.3390/jcm10081715. J Clin Med. 2021. PMID: 33923385 Free PMC article. Review.
Cited by
-
Significance of Identifying Key Genes Involved in HBV-Related Hepatocellular Carcinoma for Primary Care Surveillance of Patients with Cirrhosis.Genes (Basel). 2022 Dec 10;13(12):2331. doi: 10.3390/genes13122331. Genes (Basel). 2022. PMID: 36553600 Free PMC article.
-
EZH2-mediated epigenetic silencing of tumor-suppressive let-7c/miR-99a cluster by hepatitis B virus X antigen enhances hepatocellular carcinoma progression and metastasis.Cancer Cell Int. 2023 Sep 9;23(1):199. doi: 10.1186/s12935-023-03002-9. Cancer Cell Int. 2023. PMID: 37689710 Free PMC article.
-
Hepatitis B x (HBx) as a Component of a Functional Cure for Chronic Hepatitis B.Biomedicines. 2022 Sep 7;10(9):2210. doi: 10.3390/biomedicines10092210. Biomedicines. 2022. PMID: 36140311 Free PMC article. Review.
-
Hepatitis B virus X protein promotes MAN1B1 expression by enhancing stability of GRP78 via TRIM25 to facilitate hepatocarcinogenesis.Br J Cancer. 2023 Apr;128(6):992-1004. doi: 10.1038/s41416-022-02115-8. Epub 2023 Jan 12. Br J Cancer. 2023. PMID: 36635499 Free PMC article.
-
SMC5/6-Mediated Transcriptional Regulation of Hepatitis B Virus and Its Therapeutic Potential.Viruses. 2024 Oct 25;16(11):1667. doi: 10.3390/v16111667. Viruses. 2024. PMID: 39599784 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

