11C-radiolabeled aptamer for imaging of tumors and metastases using positron emission tomography- computed tomography
- PMID: 34853715
- PMCID: PMC8601970
- DOI: 10.1016/j.omtn.2021.10.020
11C-radiolabeled aptamer for imaging of tumors and metastases using positron emission tomography- computed tomography
Abstract
Identification of primary tumors and metastasis sites is an essential step in cancer diagnostics and the following treatment. Positron emission tomography-computed tomography (PET/CT) is one of the most reliable methods for scanning the whole organism for malignancies. In this work, we synthesized an 11C-labeled oligonucleotide primer and hybridized it to an anti-cancer DNA aptamer. The 11C-aptamer was applied for in vivo imaging of Ehrlich ascites carcinoma and its metastases in mice using PET/CT. The imaging experiments with the 11C-aptamer determined very small primary and secondary tumors of 3 mm2 and less. We also compared 11C imaging with the standard radiotracer, 2-deoxy-2-[fluorine-18]fluoro-D-glucose (18F-FDG), and found better selectivity of the 11C-aptamer to metastatic lesions in the metabolically active organs than 18F-FDG. 11C radionuclide with an ultra-short (20.38 min) half-life is considered safest for PET/CT imaging and does not cause false-positive results in heart imaging. Its combination with aptamers gives us high-specificity and high-contrast imaging of cancer cells and can be applied for PET/CT-guided drug delivery in cancer therapies.
Keywords: 11C radiolabeling; DNA aptamers; Ehrlich ascites carcinoma; PET/CT; in vivo imaging; metastasis; radiopharmaceuticals.
© 2021 The Author(s).
Conflict of interest statement
Authors declare no competing interests.
Figures

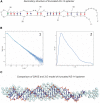

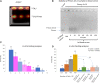


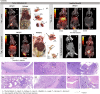
References
-
- Wafaie A.M., Moussa K.M., Ebeid E.M. Cancer of unknown primary origin: can FDG PET/CT have a role in detecting the site of primary? Egypt. J. Radiol. Nucl. Med. 2018;49:190–195.
-
- Bollineni V.R., Kramer G.M., Jansma E.P., Liu Y., Oyen W.J.G. A systematic review on [18F]FLT-PET uptake as a measure of treatment response in cancer patients. Eur. J. Cancer. 2016;55:81–97. - PubMed
-
- Positron Emission Tomography: A Guide for Clinicians (2014). (Springer Berlin Heidelberg)
LinkOut - more resources
Full Text Sources

