The Children's Anti-inflammatory Reliever (CARE) study: a protocol for a randomised controlled trial of budesonide-formoterol as sole reliever therapy in children with mild asthma
- PMID: 34853785
- PMCID: PMC8628747
- DOI: 10.1183/23120541.00271-2021
The Children's Anti-inflammatory Reliever (CARE) study: a protocol for a randomised controlled trial of budesonide-formoterol as sole reliever therapy in children with mild asthma
Abstract
Background: Asthma is the most common chronic disease in children, many of whom are managed solely with a short-acting β2-agonist (SABA). In adults, the evidence that budesonide-formoterol as sole reliever therapy markedly reduces the risk of severe exacerbations compared with SABA alone has contributed to the Global Initiative for Asthma recommending against SABA monotherapy in this population. The current lack of evidence in children means it is unknown whether these findings are also relevant to this demographic. High-quality randomised controlled trials (RCTs) are needed.
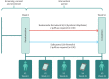
Objective: The aim of this study is to determine the efficacy and safety of as-needed budesonide-formoterol therapy compared with as-needed salbutamol in children aged 5 to 15 years with mild asthma, who only use a SABA.
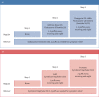
Methods: A 52-week, open-label, parallel group, phase III RCT will recruit 380 children aged 5 to 15 years with mild asthma. Participants will be randomised 1:1 to either budesonide-formoterol (Symbicort Rapihaler®) 50/3 µg, two actuations as needed, or salbutamol (Ventolin®) 100 µg, two actuations as needed. The primary outcome is asthma attacks as rate per participant per year. Secondary outcomes assess asthma control, lung function, exhaled nitric oxide and treatment step change. A cost-effectiveness analysis is also planned.
Conclusion: This is the first RCT to assess the safety and efficacy of as-needed budesonide-formoterol in children with mild asthma. The results will provide a much-needed evidence base for the treatment of mild asthma in children.
Copyright ©The authors 2021.
Conflict of interest statement
Conflict of interest: L. Hatter has nothing to disclose. Conflict of interest: P. Bruce has nothing to disclose. Conflict of interest: M. Holliday has nothing to disclose. Conflict of interest: A.J. Anderson has nothing to disclose. Conflict of interest: I. Braithwaite has nothing to disclose. Conflict of interest: A. Corin has nothing to disclose. Conflict of interest: A. Eathorne has nothing to disclose. Conflict of interest: A. Grimes has nothing to disclose. Conflict of interest: M. Harwood has nothing to disclose. Conflict of interest: T. Hills reports financial support for the present manuscript from the Health Research Council for New Zealand, by way of a research grant. Conflict of interest: C. Kearns has nothing to disclose. Conflict of interest: K. Kerse has nothing to disclose. Conflict of interest: J. Martindale has nothing to disclose. Conflict of interest: B. Montgomery has nothing to disclose. Conflict of interest: L. Riggs has nothing to disclose. Conflict of interest: D. Sheahan has nothing to disclose. Conflict of interest: N. Shortt reports financial and nonfinancial support for the present manuscript from HRC (NZ), Cure Kids and AstraZeneca. Conflict of interest: K. Zazulia has nothing to disclose. Conflict of interest: M. Weatherall has nothing to disclose. Conflict of interest: D. McNamara has nothing to disclose. Conflict of interest: C.A. Byrnes reports grants from the Health Research Council, FluLab, Curekids, and the National Health and Medical Research Council, outside the submitted work; and is an active Editorial Board member for the NZ Formulary for Children. Conflict of interest: A. Bush has nothing to disclose. Conflict of interest: S.R. Dalziel reports financial and nonfinancial support for the present manuscript from HRC (NZ), Cure Kids and AstraZeneca. Conflict of interest: R. Beasley reports financial and nonfinancial support for the present manuscript from HRC (NZ), Cure Kids and AstraZeneca; research funding from AstraZeneca and Genentech, and payment or honoraria received from AstraZeneca, Cipla, Avillion and Theravance, outside the submitted work; and a leadership or fiduciary role in the Asthma and Respiratory Foundation (NZ), outside the submitted work.
Figures
References
LinkOut - more resources
Full Text Sources
