Selective sodium iodide symporter (NIS) genetherapy of glioblastoma mediatedby EGFR-targeted lipopolyplexes
- PMID: 34853814
- PMCID: PMC8604759
- DOI: 10.1016/j.omto.2021.10.011
Selective sodium iodide symporter (NIS) genetherapy of glioblastoma mediatedby EGFR-targeted lipopolyplexes
Abstract
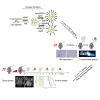
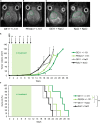
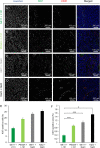
Lipo-oligomers, post-functionalized with ligands to enhance targeting, represent promising new vehicles for the tumor-specific delivery of therapeutic genes such as the sodium iodide symporter (NIS). Due to its iodide trapping activity, NIS is a powerful theranostic tool for diagnostic imaging and the application of therapeutic radionuclides. 124I PET imaging allows non-invasive monitoring of the in vivo biodistribution of functional NIS expression, and application of 131I enables cytoreduction. In our experimental design, we used epidermal growth factor receptor (EGFR)-targeted polyplexes (GE11) initially characterized in vitro using 125I uptake assays. Mice bearing an orthotopic glioblastoma were treated subsequently with mono-dibenzocyclooctyne (DBCO)-PEG24-GE11/NIS or bisDBCO-PEG24-GE11/NIS, and 24-48 h later, 124I uptake was assessed by positron emission tomography (PET) imaging. The best-performing polyplex in the imaging studies was then selected for 131I therapy studies. The in vitro studies showed EGFR-dependent and NIS-specific transfection efficiency of the polyplexes. The injection of monoDBCO-PEG24-GE11/NIS polyplexes 48 h before 124I application was characterized to be the optimal regime in the imaging studies and was therefore used for an 131I therapy study, showing a significant decrease in tumor growth and a significant extension of survival in the therapy group. These studies demonstrate the potential of EGFR-targeted polyplex-mediated NIS gene therapy as a new strategy for the therapy of glioblastoma.
Keywords: DNA nanoparticle; EGFR-targeting; GBM; NIS; gene therapy; glioblastoma; polyplexes; radioiodine; sodium iodide symporter.
© 2021 The Author(s).
Conflict of interest statement
The authors have declared no conflict of interest.
Figures




Similar articles
-
The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy.EJNMMI Res. 2022 May 3;12(1):25. doi: 10.1186/s13550-022-00888-w. EJNMMI Res. 2022. PMID: 35503582 Free PMC article. Review.
-
Imaging and targeted therapy of pancreatic ductal adenocarcinoma using the theranostic sodium iodide symporter (NIS) gene.Oncotarget. 2017 May 16;8(20):33393-33404. doi: 10.18632/oncotarget.16499. Oncotarget. 2017. PMID: 28380420 Free PMC article.
-
Dual EGFR- and TfR-targeted gene transfer for sodium iodide symporter gene therapy of glioblastoma.Mol Ther Oncolytics. 2022 Nov 3;27:272-287. doi: 10.1016/j.omto.2022.10.013. eCollection 2022 Dec 15. Mol Ther Oncolytics. 2022. PMID: 36458201 Free PMC article.
-
Reintroducing the Sodium-Iodide Symporter to Anaplastic Thyroid Carcinoma.Thyroid. 2017 Dec;27(12):1534-1543. doi: 10.1089/thy.2017.0290. Epub 2017 Nov 10. Thyroid. 2017. PMID: 29032724
-
The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment.Endocr Relat Cancer. 2021 Sep 3;28(10):T193-T213. doi: 10.1530/ERC-21-0177. Endocr Relat Cancer. 2021. PMID: 34259647 Review.
Cited by
-
Image-Guided Mesenchymal Stem Cell Sodium Iodide Symporter (NIS) Radionuclide Therapy for Glioblastoma.Cancers (Basel). 2024 Aug 20;16(16):2892. doi: 10.3390/cancers16162892. Cancers (Basel). 2024. PMID: 39199662 Free PMC article. Review.
-
Recent developments in translational imaging of in vivo gene therapy outcomes.Mol Ther. 2025 Jun 4;33(6):2548-2564. doi: 10.1016/j.ymthe.2024.12.049. Epub 2024 Dec 30. Mol Ther. 2025. PMID: 39741403 Review.
-
The sodium iodide symporter (NIS) as theranostic gene: its emerging role in new imaging modalities and non-viral gene therapy.EJNMMI Res. 2022 May 3;12(1):25. doi: 10.1186/s13550-022-00888-w. EJNMMI Res. 2022. PMID: 35503582 Free PMC article. Review.
-
Nanosystems for gene therapy targeting brain damage caused by viral infections.Mater Today Bio. 2022 Dec 17;18:100525. doi: 10.1016/j.mtbio.2022.100525. eCollection 2023 Feb. Mater Today Bio. 2022. PMID: 36619201 Free PMC article. Review.
-
Interleukin-6-controlled, mesenchymal stem cell-based sodium/iodide symporter gene therapy improves survival of glioblastoma-bearing mice.Mol Ther Oncolytics. 2023 Aug 15;30:238-253. doi: 10.1016/j.omto.2023.08.004. eCollection 2023 Sep 21. Mol Ther Oncolytics. 2023. PMID: 37701849 Free PMC article.
References
-
- Aum D.J., Kim D.H., Beaumont T.L., Leuthardt E.C., Dunn G.P., Kim A.H. Molecular and cellular heterogeneity: the hallmark of glioblastoma. Neurosurg. Focus. 2014;37:E11. - PubMed
-
- Louis D.N., Perry A., Reifenberger G., von Deimling A., Figarella-Branger D., Cavenee W.K., Ohgaki H., Wiestler O.D., Kleihues P., Ellison D.W. The 2016 world health organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. - PubMed
-
- Bastien J.I.L., McNeill K.A., Fine H.A. Molecular characterizations of glioblastoma, targeted therapy, and clinical results to date. Cancer. 2015;121:502–516. - PubMed
-
- Ginn S.L., Amaya A.K., Alexander I.E., Edelstein M., Abedi M.R. Gene therapy clinical trials worldwide to 2017: an update. J. Gene Med. 2018;20:e3015. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

