Increasing Efficacy of Enveloped Whole-Virus Vaccines by In situ Immune-Complexing with the Natural Anti-Gal Antibody
- PMID: 34853815
- PMCID: PMC8631339
- DOI: 10.18103/mra.v9i7.2481
Increasing Efficacy of Enveloped Whole-Virus Vaccines by In situ Immune-Complexing with the Natural Anti-Gal Antibody
Abstract
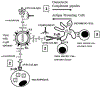
The appearance of variants of mutated virus in course of the Covid-19 pandemic raises concerns regarding the risk of possible formation of variants that can evade the protective immune response elicited by the single antigen S-protein gene-based vaccines. This risk may be avoided by inclusion of several antigens in vaccines, so that a variant that evades the immune response to the S-protein of SARS-CoV-2 virus will be destroyed by the protective immune response against other viral antigens. A simple way for preparing multi-antigenic enveloped-virus vaccines is using the inactivated whole-virus as vaccine. However, immunogenicity of such vaccines may be suboptimal because of poor uptake of the vaccine by antigen-presenting-cells (APC) due to electrostatic repulsion by the negative charges of sialic-acid on both the glycan-shield of the vaccinating virus and on the carbohydrate-chains (glycans) of APC. In addition, glycan-shield can mask many antigenic peptides. These effects of the glycan-shield can be reduced and immunogenicity of the vaccinating virus markedly increased by glycoengineering viral glycans for replacing sialic-acid units on glycans with α-gal epitopes (Galα1-3Galβ1-4GlcNAc-R). Vaccination of humans with inactivated whole-virus presenting α-gal epitopes (virusα-gal) results in formation of immune-complexes with the abundant natural anti-Gal antibody that binds to viral α-gal epitopes at the vaccination site. These immune-complexes are targeted to APC for rigorous uptake due to binding of the Fc portion of immunecomplexed anti-Gal to Fcγ receptors on APC. The APC further transport the large amounts of internalized vaccinating virus to regional lymph nodes, process and present the virus antigenic peptides for the activation of many clones of virus specific helper and cytotoxic T-cells. This elicits a protective cellular and humoral immune response against multiple viral antigens and an effective immunological memory. The immune response to virusα-gal vaccine was studied in mice producing anti-Gal and immunized with inactivated influenza-virusα-gal. These mice demonstrated 100-fold increase in titer of the antibodies produced, a marked increase in T-cell response, and a near complete protection against challenge with a lethal dose of live influenza-virus, in comparison to a similar vaccine lacking α-gal epitopes. This glycoengineering can be achieved in vitro by enzymatic reaction with neuraminidase removing sialic-acid and with recombinant α1,3galactosyltransferase (α1,3GT) synthesizing α-gal epitopes, by engineering host-cells to contain several copies of the α1,3GT gene (GGTA1), or by transduction of this gene in a replication-defective adenovirus vector into host-cells. Theoretically, these methods for increased immunogenicity may be applicable to all enveloped viruses with N-glycans on their envelope.
Keywords: Inactivated whole-virus vaccine; enveloped virus vaccines; glycan-shield; natural anti-Gal antibody; vaccine immunogenicity; variants; α-gal epitope.
Figures
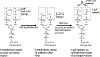

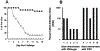
Similar articles
-
Amplifying immunogenicity of prospective Covid-19 vaccines by glycoengineering the coronavirus glycan-shield to present α-gal epitopes.Vaccine. 2020 Sep 29;38(42):6487-6499. doi: 10.1016/j.vaccine.2020.08.032. Epub 2020 Aug 19. Vaccine. 2020. PMID: 32907757 Free PMC article. Review.
-
The alpha-gal epitope and the anti-Gal antibody in xenotransplantation and in cancer immunotherapy.Immunol Cell Biol. 2005 Dec;83(6):674-86. doi: 10.1111/j.1440-1711.2005.01366.x. Immunol Cell Biol. 2005. PMID: 16266320 Review.
-
Immunogenicity of influenza virus vaccine is increased by anti-gal-mediated targeting to antigen-presenting cells.J Virol. 2007 Sep;81(17):9131-41. doi: 10.1128/JVI.00647-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609270 Free PMC article.
-
Increased immunogenicity of tumor vaccines complexed with anti-Gal: studies in knockout mice for alpha1,3galactosyltransferase.Cancer Res. 1999 Jul 15;59(14):3417-23. Cancer Res. 1999. PMID: 10416604
-
Increased immunogenicity of human immunodeficiency virus gp120 engineered to express Galalpha1-3Galbeta1-4GlcNAc-R epitopes.J Virol. 2006 Jul;80(14):6943-51. doi: 10.1128/JVI.00310-06. J Virol. 2006. PMID: 16809300 Free PMC article.
Cited by
-
Approaching Challenges Posed by SARS-CoV-2 Genetic Variants.Pathogens. 2022 Nov 23;11(12):1407. doi: 10.3390/pathogens11121407. Pathogens. 2022. PMID: 36558741 Free PMC article.
-
Evolution of tick vaccinology.Parasitology. 2024 Aug;151(9):1045-1052. doi: 10.1017/S003118202400043X. Epub 2024 Apr 8. Parasitology. 2024. PMID: 38586999 Free PMC article. Review.
-
Quantum vaccinomics platforms to advance in vaccinology.Front Immunol. 2023 Jun 15;14:1172734. doi: 10.3389/fimmu.2023.1172734. eCollection 2023. Front Immunol. 2023. PMID: 37398646 Free PMC article.
References
-
- Lauring AS, Hodcroft EB. Genetic Variants of SARS-CoV-2-What Do They Mean? JAMA. 2021;325:529–531. - PubMed
-
- Borrow P, Lewicki H, Wei X, et al. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997; 3:205–211. - PubMed
-
- Wei X, Decker JM, Wang S, et al. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. - PubMed
-
- Leonard CK, Spellman MW, Riddle L, Harris RJ, Thomas JN, Gregory TJ. Assignment of intrachain disulfide bonds and characterization of potential glycosylation sites of the type 1 recombinant human immunodeficiency virus envelope glycoprotein (gp120) expressed in Chinese hamster ovary cells. J Biol Chem 1990;265:10373–10382. - PubMed
-
- Mizuochi T, Matthews T, Kato M, et al. Diversity of oligosaccharide structures on the envelope glycoprotein gp120 of human immunodeficiency virus 1 from the lymphoblastoid cell line H9. Presence of complex-type oligosaccharides with bisecting N-acetylglucosamine residues. J Biol Chem. 1990;265:8519–8524. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
