Anti-drug Antibody Validation Testing and Reporting Harmonization
- PMID: 34853961
- PMCID: PMC8816448
- DOI: 10.1208/s12248-021-00649-y
Anti-drug Antibody Validation Testing and Reporting Harmonization
Abstract
Evolving immunogenicity assay performance expectations and a lack of harmonized anti-drug antibody validation testing and reporting tools have resulted in significant time spent by health authorities and sponsors on resolving filing queries. Following debate at the American Association of Pharmaceutical Sciences National Biotechnology Conference, a group was formed to address these gaps. Over the last 3 years, 44 members from 29 organizations (including 5 members from Europe and 10 members from FDA) discussed gaps in understanding immunogenicity assay requirements and have developed harmonization tools for use by industry scientists to facilitate filings to health authorities. Herein, this team provides testing and reporting strategies and tools for the following assessments: (1) pre-study validation cut point; (2) in-study cut points, including procedures for applying cut points to mixed populations; (3) system suitability control criteria for in-study plate acceptance; (4) assay sensitivity, including the selection of an appropriate low positive control; (5) specificity, including drug and target tolerance; (6) sample stability that reflects sample storage and handling conditions; (7) assay selectivity to matrix components, including hemolytic, lipemic, and disease state matrices; (8) domain specificity for multi-domain therapeutics; (9) and minimum required dilution and extraction-based sample processing for titer reporting.
Keywords: Anti-drug antibodies (ADA); FDA; Immunogenicity; Neutralizing antibodies (NAb); Regulatory guidance; Validation.
© 2021. The Author(s).
Figures
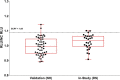
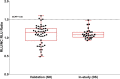
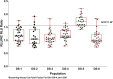
References
-
- (USP) USP. General chapter <1106> Immunogenicity assays - design and validation of immunogenicity assays to detect anti-drug antibodies. 2014.
-
- Food US, Drug A. Guidance for industry: immunogenicity testing of therapeutic protein products - developing and validating assays for anti-drug antibody detection. Rockville: US Food and Drug Administration. 2019.
-
- EMA. Guideline on immunogenicity assessment of therapeutic proteins. In: (CHMP) CfMPfHU, editor. London. 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

