Real-World Safety and Effectiveness of Canagliflozin Treatment for Type 2 Diabetes Mellitus in Japan: SAPPHIRE, a Long-Term, Large-Scale Post-Marketing Surveillance
- PMID: 34853985
- PMCID: PMC8799573
- DOI: 10.1007/s12325-021-01984-4
Real-World Safety and Effectiveness of Canagliflozin Treatment for Type 2 Diabetes Mellitus in Japan: SAPPHIRE, a Long-Term, Large-Scale Post-Marketing Surveillance
Abstract
Introduction: This long-term post-marketing surveillance (SAPPHIRE) collected information on the safety and effectiveness of canagliflozin (approved dose 100 mg) prescribed to patients with type 2 diabetes mellitus (T2DM) in real-world practice in Japan.
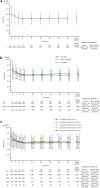
Methods: Patients with T2DM who were prescribed canagliflozin between December 2014 and September 2016 were registered and observed for up to 3 years. Safety was evaluated in terms of adverse drug reactions (ADRs). Effectiveness was assessed in terms of glycaemic control. Data were also analysed across age subgroups (< 65, ≥ 65 to < 75, and ≥ 75 years old) and the estimated glomerular filtration rate (eGFR) categories for chronic kidney disease (G1-G5 based on eGFR) at baseline.
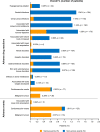
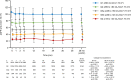
Results: A total of 12,227 patients were included in the safety analyses and 11,675 in effectiveness analyses. Overall, 7104 patients were treated with canagliflozin for ≥ 3 years. The mean age, haemoglobin A1c (HbA1c), and eGFR at baseline were 58.4 ± 12.5 years, 8.01 ± 1.49%, and 80.04 ± 21.85 mL/min/1.73 m2, respectively. There were 1836 ADRs in 1312 patients (10.73%) and 268 serious ADRs in 225 patients (1.84%). The most common ADRs were those related to volume depletion (1.39%), genital infection (1.34%), polyuria/pollakiuria (1.23%), and urinary tract infection (1.19%). The frequencies of ADRs tended to increase with age and stage of chronic kidney disease. The reductions in mean HbA1c after starting canagliflozin were maintained for up to 3 years with a mean change of - 0.68% (n = 6345 at 3 years). Maintained reductions in mean HbA1c were observed in each age subgroup and in patients with G1-G3b renal function.
Conclusion: This surveillance in real-world clinical practice showed that canagliflozin provides sustained glucose-lowering effects in patients with T2DM, including elderly patients and patients with moderate renal impairment, without new safety concerns beyond those already described in the Japanese package insert.
Trial registration: JapicCTI-153048.
Keywords: Canagliflozin; Japan; Post-marketing surveillance; Real-world; Safety; Sodium–glucose cotransporter 2 inhibitor; Type 2 diabetes mellitus.
Plain language summary
Canagliflozin is a sodium–glucose cotransporter 2 (SGLT2) inhibitor that lowers blood glucose levels by increasing urinary glucose excretion. It was approved for the management of blood glucose levels in patients with type 2 diabetes mellitus following clinical trials. However, clinical trials may not fully represent the safety or effectiveness of a drug in real-world clinical practice. Therefore, a 3-year post-marketing surveillance was performed in Japan to obtain safety and effectiveness data for a large group of 12,227 patients with type 2 diabetes mellitus and various demographic/clinical characteristics. Safety and effectiveness data were collected for up to 3 years while patients were treated with canagliflozin. Adverse drug reactions occurred in 10.73% of patients. The most common types of adverse drug reactions were those related to volume depletion (body fluid decreased), followed by genital infection, polyuria/pollakiuria (increased urination), and urinary tract infection. Adverse drug reactions tended to be more common in elderly patients and in patients with renal impairment. As expected, canagliflozin was associated with improvements in haemoglobin A1c, a marker of blood glucose control, in patients with type 2 diabetes, including in elderly patients and patients with moderate renal impairment. In this surveillance in real-world clinical practice, long-term treatment with canagliflozin raised no new safety concerns beyond the information already included in the Japanese package insert. Canagliflozin provides sustained glucose-lowering effects.
© 2021. The Author(s).
Figures

Similar articles
-
Real-World Evidence of Treatment with Teneligliptin/Canagliflozin Combination Tablets for Type 2 Diabetes Mellitus: A Post-Marketing Surveillance in Japan.Adv Ther. 2022 Apr;39(4):1642-1658. doi: 10.1007/s12325-021-02038-5. Epub 2022 Feb 9. Adv Ther. 2022. PMID: 35138572 Free PMC article.
-
Long-Term Safety and Efficacy of Teneligliptin in Elderly Patients with Type 2 Diabetes: Subgroup Analysis of a 3-Year Post-Marketing Surveillance in Japan.Adv Ther. 2020 May;37(5):2477-2492. doi: 10.1007/s12325-020-01306-0. Epub 2020 Apr 22. Adv Ther. 2020. PMID: 32323194 Free PMC article.
-
Long-Term, Real-World Safety and Efficacy of Teneligliptin: A Post-Marketing Surveillance of More Than 10,000 Patients with Type 2 Diabetes in Japan.Adv Ther. 2020 Mar;37(3):1065-1086. doi: 10.1007/s12325-019-01189-w. Epub 2019 Dec 23. Adv Ther. 2020. PMID: 31873865 Free PMC article.
-
Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus.Drugs. 2015 Jan;75(1):33-59. doi: 10.1007/s40265-014-0337-y. Drugs. 2015. PMID: 25488697 Review.
-
A review of the efficacy and safety of canagliflozin in elderly patients with type 2 diabetes.Consult Pharm. 2014;29(5):335-46. doi: 10.4140/TCP.n.2014.335. Consult Pharm. 2014. PMID: 24849690 Review.
Cited by
-
Treatment patterns and satisfaction in patients with type 2 diabetes newly initiating oral monotherapy with antidiabetic drugs in Japan: results from the prospective Real-world Observational Study on Patient Outcomes in Diabetes (RESPOND).BMJ Open Diabetes Res Care. 2022 Dec;10(6):e003032. doi: 10.1136/bmjdrc-2022-003032. BMJ Open Diabetes Res Care. 2022. PMID: 36585033 Free PMC article.
-
Safety and effectiveness of tofogliflozin in Japanese people with type 2 diabetes: A multicenter prospective observational study in routine clinical practice.J Diabetes Investig. 2024 Nov;15(11):1585-1595. doi: 10.1111/jdi.14287. Epub 2024 Aug 14. J Diabetes Investig. 2024. PMID: 39141404 Free PMC article.
-
Predictive Factors of Renal Function Decline in Patients with Type 2 Diabetes Treated with Canagliflozin in the Real-Wecan Study.J Clin Med. 2022 Sep 24;11(19):5622. doi: 10.3390/jcm11195622. J Clin Med. 2022. PMID: 36233490 Free PMC article.
-
Comparison over Time of Adverse Drug Reactions in Diabetes Patients Treated with Sodium-Glucose Cotransporter 2 Inhibitors.Kobe J Med Sci. 2024 Jul 25;70(3):E81-E88. doi: 10.24546/0100490464. Kobe J Med Sci. 2024. PMID: 39107964 Free PMC article.
-
Comparative safety of different sodium-glucose transporter 2 inhibitors in patients with type 2 diabetes: a systematic review and network meta-analysis of randomized controlled trials.Front Endocrinol (Lausanne). 2023 Aug 28;14:1238399. doi: 10.3389/fendo.2023.1238399. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37701900 Free PMC article.
References
-
- Mitsubishi Tanabe Pharma Corporation. [CANAGLU® 100 mg tablets. revised June 2019] https://medical.mt-pharma.co.jp/di/file/dc/can_a.pdf. Accessed 8 June 2021 (In Japanese).
-
- Inagaki N, Nangaku M, Sakata Y, et al. Safety and efficacy of canagliflozin, a sodium-glucose co-transporter 2 inhibitor, as monotherapy and in combination with antidiabetic agents in patients with type 2 diabetes mellitus – an interim analysis of post-marketing surveillance (SAPPHIRE) Jpn Pharmacol Ther. 2018;46(4):499–519.
-
- Inagaki N, Nangaku M, Sakata Y, et al. Safety and efficacy of canagliflozin, an SGLT2 inhibitor, in patients with type 2 diabetes mellitus – an interim analysis of post-marketing surveillance (SAPPHIRE) J New Remed Clin. 2019;68(1):9–37.
-
- Inagaki N, Nangaku M, Sakata Y, Sasaki K, Nakamura H, Hamada K. Safety and efficacy of canagliflozin, an SGLT2 inhibitor, in patients with type 2 diabetes mellitus – the interim analysis of post-marketing surveillance (SAPPHIRE), third report. J New Remed Clin. 2020;69(3):288–329.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

