Genetic deletion or pharmacological blockade of nociceptin/orphanin FQ receptors in the ventral tegmental area attenuates nicotine-motivated behaviour
- PMID: 34854073
- PMCID: PMC9081114
- DOI: 10.1111/bph.15762
Genetic deletion or pharmacological blockade of nociceptin/orphanin FQ receptors in the ventral tegmental area attenuates nicotine-motivated behaviour
Abstract
Background and purpose: The nociceptin/orphanin FQ (N/OFQ)-nociceptin opioid-like peptide (NOP) receptor system is widely distributed in the brain and pharmacological activation of this system revealed therapeutic potential in animal models of substance use disorder. Studies also showed that genetic deletion or pharmacological blockade of NOP receptors confer resistance to the development of alcohol abuse. Here, we have used a genetic and pharmacological approach to evaluate the therapeutic potential of NOP antagonism in smoking cessation.
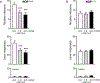
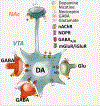
Experimental approach: Constitutive NOP receptor knockout rats (NOP-/- ) and their wild-type counterparts (NOP+/+ ) were tested over a range of behaviours to characterize their motivation for nicotine. We next explored the effects of systemic administration of the NOP receptor antagonist LY2817412 (1.0 & 3.0 mg·kg-1 ) on nicotine self-administration. NOP receptor blockade was further evaluated at the brain circuitry level, by microinjecting LY2817412 (3.0 & 6.0 μg·μl-1 ) into the ventral tegmental area (VTA), nucleus accumbens (NAc) and central amygdala (CeA).
Key results: Genetic NOP receptor deletion resulted in decreased nicotine intake, decreased motivation to self-administer and attenuation of cue-induced nicotine reinstatement. LY2817412 reduced nicotine intake in NOP+/+ but not in NOP-/- rats, confirming that its effect is mediated by inhibition of NOP transmission. Finally, injection of LY2817412 into the VTA but not into the NAc or CeA decreased nicotine self-administration.
Conclusions and implications: These findings indicate that inhibition of NOP transmission attenuates the motivation for nicotine through mechanisms involving the VTA and suggest that NOP receptor antagonism may represent a potential treatment for smoking cessation.
Keywords: NOP; VTA; nicotine; reinforcement; relapse; reward.
© 2021 The British Pharmacological Society.
Conflict of interest statement
Figures
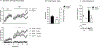

Similar articles
-
Pharmacological blockage of NOP receptors decreases ventral tegmental area dopamine neuronal activity through GABAB receptor-mediated mechanism.Neuropharmacology. 2024 May 1;248:109866. doi: 10.1016/j.neuropharm.2024.109866. Epub 2024 Feb 15. Neuropharmacology. 2024. PMID: 38364970 Free PMC article.
-
NOP receptor antagonism reduces alcohol drinking in male and female rats through mechanisms involving the central amygdala and ventral tegmental area.Br J Pharmacol. 2020 Apr;177(7):1525-1537. doi: 10.1111/bph.14915. Epub 2020 Feb 3. Br J Pharmacol. 2020. PMID: 31713848 Free PMC article.
-
NOP receptor antagonism attenuates reinstatement of alcohol-seeking through modulation of the mesolimbic circuitry in male and female alcohol-preferring rats.Neuropsychopharmacology. 2021 Nov;46(12):2121-2131. doi: 10.1038/s41386-021-01096-1. Epub 2021 Jul 20. Neuropsychopharmacology. 2021. PMID: 34285372 Free PMC article.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
-
Nociceptin/orphanin FQ receptor antagonists as innovative antidepressant drugs.Pharmacol Ther. 2013 Oct;140(1):10-25. doi: 10.1016/j.pharmthera.2013.05.008. Epub 2013 May 24. Pharmacol Ther. 2013. PMID: 23711793 Review.
Cited by
-
Epigenetic Modulation of Opioid Receptors by Drugs of Abuse.Int J Mol Sci. 2022 Oct 5;23(19):11804. doi: 10.3390/ijms231911804. Int J Mol Sci. 2022. PMID: 36233105 Free PMC article. Review.
-
Nicotine but not saline self-administering or yoked control conditions produces sustained neuroadaptations in the accumbens shell.Front Mol Neurosci. 2023 Jan 25;16:1105388. doi: 10.3389/fnmol.2023.1105388. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36760603 Free PMC article.
-
Abstinence-Induced Nicotine Seeking Relays on a Persistent Hypoglutamatergic State within the Amygdalo-Striatal Neurocircuitry.eNeuro. 2023 Feb 21;10(2):ENEURO.0468-22.2023. doi: 10.1523/ENEURO.0468-22.2023. Print 2023 Feb. eNeuro. 2023. PMID: 36754627 Free PMC article.
-
Regulation of N-type calcium channels by nociceptin receptors and its possible role in neurological disorders.Mol Brain. 2022 Nov 24;15(1):95. doi: 10.1186/s13041-022-00982-z. Mol Brain. 2022. PMID: 36434658 Free PMC article. Review.
-
Pharmacological blockage of NOP receptors decreases ventral tegmental area dopamine neuronal activity through GABAB receptor-mediated mechanism.Neuropharmacology. 2024 May 1;248:109866. doi: 10.1016/j.neuropharm.2024.109866. Epub 2024 Feb 15. Neuropharmacology. 2024. PMID: 38364970 Free PMC article.
References
-
- Alexander SP, Christopoulos A, Davenport AP, Kelly E, Mathie A, Peters JA, et al. (2021). THE CONCISE GUIDE TO PHARMACOLOGY 2021/22: G protein-coupled receptors. British Journal of Pharmacology 178: S27–S156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

