Metronidazole gel (0.75%) in Japanese patients with rosacea: A randomized, vehicle-controlled, phase 3 study
- PMID: 34854112
- PMCID: PMC9299697
- DOI: 10.1111/1346-8138.16254
Metronidazole gel (0.75%) in Japanese patients with rosacea: A randomized, vehicle-controlled, phase 3 study
Abstract
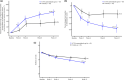
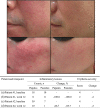
Topical metronidazole is not currently approved in Japan as a treatment for the indication of rosacea, although 0.75% metronidazole gel was authorized in 2014 for the management of cancerous skin ulcers. We conducted a randomized, double-blind, vehicle-controlled study to evaluate the efficacy and safety of 0.75% metronidazole gel in Japanese patients with inflammatory lesions (papules/pustules) and erythema associated with moderate to severe rosacea. Overall, 130 patients were randomly assigned to receive 0.75% metronidazole gel (n = 65) or vehicle (n = 65), and 120 patients completed 12 weeks of treatment. The primary efficacy outcome was the proportion of patients who achieved both of the following at week 12: an improvement of >50% in the number of inflammatory lesions (papules/pustules) and a positive change of at least one degree in erythema severity. This composite outcome was achieved by 72.3% of metronidazole-treated patients versus 36.9% of vehicle-treated patients, with the between-group difference demonstrating significant improvement with 0.75% metronidazole gel (p < 0.0001). All secondary efficacy endpoints (patients achieving a score of ≥3 for percent change in the number of inflammatory lesions at week 12; patients achieving a score of ≥3 for change in erythema severity at week 12; patients achieving an Investigator's Global Assessment score of 0 or 1 at week 12; percent change over time in the number of inflammatory lesions; change over time in erythema severity) also showed improvement in the 0.75% metronidazole gel group. The incidence of adverse events was higher with metronidazole (40.0%) than with vehicle (29.2%). Of these, treatment-related, treatment-emergent adverse events occurred in 9.2% and 6.2% in the metronidazole and the vehicle group, respectively, but there were no new safety concerns. Overall, the results of this study have confirmed the efficacy and safety of 0.75% metronidazole gel in Japanese patients with rosacea.
Keywords: Japanese; clinical trial, phase III; metronidazole; rosacea.
© 2021 The Authors. The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
This study was funded by Maruho. Y.M. and K.Y. have received research funding, consultancy fees, speaker fees, fees for arranging education, and personal fees from Maruho. T.F. and C.F. are employees of Maruho.
Figures
Similar articles
-
A comparison of 15% azelaic acid gel and 0.75% metronidazole gel in the topical treatment of papulopustular rosacea: results of a randomized trial.Arch Dermatol. 2003 Nov;139(11):1444-50. doi: 10.1001/archderm.139.11.1444. Arch Dermatol. 2003. PMID: 14623704 Clinical Trial.
-
Topical metronidazole maintains remissions of rosacea.Arch Dermatol. 1998 Jun;134(6):679-83. doi: 10.1001/archderm.134.6.679. Arch Dermatol. 1998. PMID: 9645635 Clinical Trial.
-
Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies.J Drugs Dermatol. 2013 Jun 1;12(6):650-6. J Drugs Dermatol. 2013. PMID: 23839181 Clinical Trial.
-
Azelaic acid 15% gel: in the treatment of papulopustular rosacea.Am J Clin Dermatol. 2004;5(1):57-64. doi: 10.2165/00128071-200405010-00009. Am J Clin Dermatol. 2004. PMID: 14979745 Review.
-
Topical metronidazole for rosacea.Skin Therapy Lett. 2002 Jan;7(1):1-3, 6. Skin Therapy Lett. 2002. PMID: 11859387 Review.
Cited by
-
Rosacea: Practical Guidance and Challenges for Clinical Management.Clin Cosmet Investig Dermatol. 2024 Jan 23;17:175-190. doi: 10.2147/CCID.S391705. eCollection 2024. Clin Cosmet Investig Dermatol. 2024. PMID: 38283794 Free PMC article. Review.
-
Role of the skin microbiota and intestinal microbiome in rosacea.Front Microbiol. 2023 Feb 10;14:1108661. doi: 10.3389/fmicb.2023.1108661. eCollection 2023. Front Microbiol. 2023. PMID: 36846769 Free PMC article. Review.
-
Perspectives on rosacea patient characteristics and quality of life using baseline data from a phase 3 clinical study conducted in Japan.J Dermatol. 2022 Dec;49(12):1221-1227. doi: 10.1111/1346-8138.16596. Epub 2022 Sep 30. J Dermatol. 2022. PMID: 36177741 Free PMC article. Clinical Trial.
-
Real-world Evidence for the Treatment of Rosacea with Sulfur or Metronidazole Preparation in Japanese Patients.JMA J. 2023 Oct 16;6(4):448-454. doi: 10.31662/jmaj.2023-0100. Epub 2023 Sep 20. JMA J. 2023. PMID: 37941711 Free PMC article.
-
Efficacy and safety of antibiotic agents in the treatment of rosacea: a systemic network meta-analysis.Front Pharmacol. 2023 May 11;14:1169916. doi: 10.3389/fphar.2023.1169916. eCollection 2023. Front Pharmacol. 2023. PMID: 37251342 Free PMC article.
References
-
- Gether L, Overgaard LK, Egeberg A, Thyssen JP. Incidence and prevalence of rosacea: a systematic review and meta‐analysis. Br J Dermatol. 2018;179:282–9. - PubMed
-
- Alexis AF, Callender VD, Baldwin HE, Desai SR, Rendon MI, Taylor SC. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: Review and clinical practice experience. J Am Acad Dermatol. 2019;80:1722–9. - PubMed
-
- Li J, Wang B, Deng Y, Shi W, Jian D, Liu F, et al. Epidemiological features of rosacea in Changsha, China: a population‐based, cross‐sectional study. J Dermatol. 2020;47:497–502. - PubMed
-
- Furue M, Yamazaki S, Jimbow K, Tsuchida T, Amagai M, Tanaka T, et al. Prevalence of dermatological disorders in Japan: a nationwide, cross‐sectional, seasonal, multicenter, hospital‐based study. J Dermatol. 2011;38:310–20. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

