Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment
- PMID: 34854206
- PMCID: PMC9168966
- DOI: 10.1002/1878-0261.13146
Dual inhibition of TGF-β and PD-L1: a novel approach to cancer treatment
Abstract
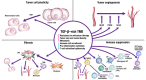
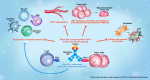
Transforming growth factor-β (TGF-β) and programmed death ligand 1 (PD-L1) initiate signaling pathways with complementary, nonredundant immunosuppressive functions in the tumor microenvironment (TME). In the TME, dysregulated TGF-β signaling suppresses antitumor immunity and promotes cancer fibrosis, epithelial-to-mesenchymal transition, and angiogenesis. Meanwhile, PD-L1 expression inactivates cytotoxic T cells and restricts immunosurveillance in the TME. Anti-PD-L1 therapies have been approved for the treatment of various cancers, but TGF-β signaling in the TME is associated with resistance to these therapies. In this review, we discuss the importance of the TGF-β and PD-L1 pathways in cancer, as well as clinical strategies using combination therapies that block these pathways separately or approaches with dual-targeting agents (bispecific and bifunctional immunotherapies) that may block them simultaneously. Currently, the furthest developed dual-targeting agent is bintrafusp alfa. This drug is a first-in-class bifunctional fusion protein that consists of the extracellular domain of the TGF-βRII receptor (a TGF-β 'trap') fused to a human immunoglobulin G1 (IgG1) monoclonal antibody blocking PD-L1. Given the immunosuppressive effects of the TGF-β and PD-L1 pathways within the TME, colocalized and simultaneous inhibition of these pathways may potentially improve clinical activity and reduce toxicity.
Keywords: PD-L1; TGF-β; immune checkpoint inhibitor; tumor microenvironment.
© 2021 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies. This article has been contributed to by US Government employees and their work is in the public domain in the USA.
Conflict of interest statement
JLG reports a collaborative research and development agreement between the National Cancer Institute (NCI) and EMD Serono, Billerica, MA, USA, a patent with the NCI entitled ‘Combination PDL1 and TGF‐beta blockade in patients with HPV+ malignancies’, unpaid membership on the Data Safety Monitoring Board for bintrafusp alfa, and an unpaid position of NCI Liaison to the Board of the Society for Immunotherapy of Cancer. J Schlom reports a collaborative research and development agreement between the NCI and EMD Serono, Billerica, MA, USA. MHBH reports grants from Roche‐Genentech, Innovation Pathways, NCI, and Varian Medical Systems; consulting fees from Telos Inc. and Innovation Pathways; honoraria from the Society for Immunotherapy in Cancer; conference chairmanship for American Association for Cancer Research; advisory committee memberships for Genentech and EMD Serono, Billerica, MA, USA; a patent entitled ‘DNA Damage Repair Deficit in Cancer Cells’; and receipt of research drugs from Innovation Pathways. FA and YL report employment at EMD Serono, Billerica, MA, USA. ID reports previous employment at EMD Serono, Billerica, MA, USA. XJW, J Seoane, and AM do not have any conflicts of interest to disclose.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

