Low-affinity integrin states have faster ligand-binding kinetics than the high-affinity state
- PMID: 34854380
- PMCID: PMC8730728
- DOI: 10.7554/eLife.73359
Low-affinity integrin states have faster ligand-binding kinetics than the high-affinity state
Abstract
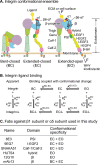
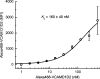
Integrin conformational ensembles contain two low-affinity states, bent-closed and extended-closed, and an active, high-affinity, extended-open state. It is widely thought that integrins must be activated before they bind ligand; however, one model holds that activation follows ligand binding. As ligand-binding kinetics are not only rate limiting for cell adhesion but also have important implications for the mechanism of activation, we measure them here for integrins α4β1 and α5β1 and show that the low-affinity states bind substantially faster than the high-affinity state. On- and off-rates are similar for integrins on cell surfaces and as ectodomain fragments. Although the extended-open conformation's on-rate is ~20-fold slower, its off-rate is ~25,000-fold slower, resulting in a large affinity increase. The tighter ligand-binding pocket in the open state may slow its on-rate. Low-affinity integrin states not only bind ligand more rapidly, but are also more populous on the cell surface than high-affinity states. Thus, our results suggest that integrin binding to ligand may precede, rather than follow, activation by 'inside-out signaling.'
Keywords: binding kinetics; biochemistry; chemical biology; cytoskeletal force; integrin α4β1; integrin α5β1; molecular biophysics; none; structural biology.
© 2021, Li et al.
Conflict of interest statement
JL, JY, TS No competing interests declared
Figures








Similar articles
-
Effect of divalent cations on the affinity and selectivity of alpha4 integrins towards the integrin ligands vascular cell adhesion molecule-1 and mucosal addressin cell adhesion molecule-1: Ca2+ activation of integrin alpha4beta1 confers a distinct ligand specificity.Cell Commun Adhes. 2002 Jul-Aug;9(4):205-19. doi: 10.1080/15419060216014. Cell Commun Adhes. 2002. PMID: 12699089
-
High integrin αVβ6 affinity reached by hybrid domain deletion slows ligand-binding on-rate.Proc Natl Acad Sci U S A. 2018 Feb 13;115(7):E1429-E1436. doi: 10.1073/pnas.1718662115. Epub 2018 Jan 29. Proc Natl Acad Sci U S A. 2018. PMID: 29378937 Free PMC article.
-
Real-time analysis of conformation-sensitive antibody binding provides new insights into integrin conformational regulation.J Biol Chem. 2009 May 22;284(21):14337-46. doi: 10.1074/jbc.M901178200. Epub 2009 Feb 27. J Biol Chem. 2009. PMID: 19251697 Free PMC article.
-
Integrin inside-out signaling and the immunological synapse.Curr Opin Cell Biol. 2012 Feb;24(1):107-15. doi: 10.1016/j.ceb.2011.10.004. Epub 2011 Nov 28. Curr Opin Cell Biol. 2012. PMID: 22129583 Free PMC article. Review.
-
Conformational regulation of integrin structure and function.Annu Rev Biophys Biomol Struct. 2002;31:485-516. doi: 10.1146/annurev.biophys.31.101101.140922. Epub 2001 Oct 25. Annu Rev Biophys Biomol Struct. 2002. PMID: 11988479 Review.
Cited by
-
An αIIbβ3 monoclonal antibody traps a semiextended conformation and allosterically inhibits large ligand binding.Blood Adv. 2024 Aug 27;8(16):4398-4409. doi: 10.1182/bloodadvances.2024013177. Blood Adv. 2024. PMID: 38968144 Free PMC article.
-
Selective inhibition of integrin αvβ6 leads to rapid induction of urinary bladder tumors in cynomolgus macaques.Toxicol Sci. 2023 Feb 17;191(2):400-413. doi: 10.1093/toxsci/kfac128. Toxicol Sci. 2023. PMID: 36515490 Free PMC article.
-
Neutralizing Antibodies Against Allosteric Proteins: Insights From a Bacterial Adhesin.J Mol Biol. 2022 Sep 15;434(17):167717. doi: 10.1016/j.jmb.2022.167717. Epub 2022 Jul 4. J Mol Biol. 2022. PMID: 35798162 Free PMC article. Review.
-
Poly(l-lactic acid) Scaffold Releasing an α4β1 Integrin Agonist Promotes Nonfibrotic Skin Wound Healing in Diabetic Mice.ACS Appl Bio Mater. 2023 Jan 16;6(1):296-308. doi: 10.1021/acsabm.2c00890. Epub 2022 Dec 21. ACS Appl Bio Mater. 2023. PMID: 36542733 Free PMC article.
-
Receptor-Ligand Binding: Effect of Mechanical Factors.Int J Mol Sci. 2023 May 21;24(10):9062. doi: 10.3390/ijms24109062. Int J Mol Sci. 2023. PMID: 37240408 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

