Screening for cervical cancer: Choices & dilemmas
- PMID: 34854432
- PMCID: PMC9131755
- DOI: 10.4103/ijmr.IJMR_857_20
Screening for cervical cancer: Choices & dilemmas
Abstract
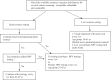
Cervical cancer is the fourth most common cancer in women in the world. To eliminate cervical cancer by 2030, the World Health Organization has given the target of 70 per cent coverage of twice lifetime screening. A multitude of screening methods are available, including cytology, human papillomavirus (HPV) DNA testing and visual inspection tests. Precision tests, including molecular and protein biomarkers such as DNA methylation, p16 immunostaining, and HPV mRNA testing help to enhance specificity of the screening. Worldwide HPV DNA testing with or without cytology is used as a screening method of choice, while in resource-poor settings, visual inspection tests are recommended. The major hurdle is a uniform and systematic implementation with a recall method in the population. Besides, controversies still exist regarding strategies to manage HPV-positive women and developing guidelines to screen the vaccinated population.
Keywords: Biomarker; DNA methylation; HPV DNA testing; VIA; cervical cancer screening; cervical cytology; p16/Ki 67 dual staining.
Conflict of interest statement
None
Figures
References
-
- Global Cancer Observatory. International Agency for Research on Cancer. [accessed on March 31, 2021]. Available from:https://gco.iarc.fr .
-
- Brisson M, Drolet M. Global elimination of cervical cancer as a public health problem. Lancet Oncol. 2019;20:319–21. - PubMed