Exploring the Influence of Intermolecular Interactions in Prebiotic Chemistry Using Laser Spectroscopy and Calculations
- PMID: 34854511
- PMCID: PMC9299682
- DOI: 10.1002/chem.202103636
Exploring the Influence of Intermolecular Interactions in Prebiotic Chemistry Using Laser Spectroscopy and Calculations
Abstract
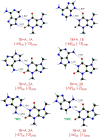
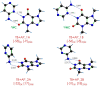
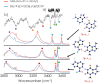
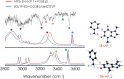
One of the most fascinating questions in chemistry is why nature chose CGAT as the alphabet of life. Very likely, such selection was the result of multiple factors and a long period of refinement. Here, we explore how the intermolecular interactions influenced such process, by characterizing the formation of dimers between adenine, theobromine and 4-aminopyrimidine. Using a combination of mass-resolved excitation spectroscopy and DFT calculations, we determined the structure of adenine-theobromine and 4-aminopyrimidine-theobromine dimers. The binding energy of these dimers is very close to the canonical adenine-thymine nucleobases. Likewise, the dimers are able to adopt Watson-Crick conformations. These findings seem to indicate that there were many options available to build the first versions of the informational polymers, which also had to compete with other molecules, such as 4-aminopyrimidine, which does not have a valid attaching point for a saccharide. For some reason, nature did not select the most strongly-bonded partners or if it did, such proto-bases were later replaced by the nowadays canonical CGAT.
Keywords: aggregation; computational chemistry; laser spectroscopy; noncovalent interactions; prebiotic chemistry.
© 2021 The Authors. Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Aggregation of nucleobases and metabolites: Adenine-theobromine trimers.J Chem Phys. 2023 Feb 14;158(6):064304. doi: 10.1063/5.0137717. J Chem Phys. 2023. PMID: 36792500
-
Probing the Watson-Crick, wobble, and sugar-edge hydrogen bond sites of uracil and thymine.J Phys Chem A. 2005 Jun 16;109(23):5055-63. doi: 10.1021/jp0446027. J Phys Chem A. 2005. PMID: 16833858
-
Noncovalent interactions in paired DNA nucleobases investigated by terahertz spectroscopy and solid-state density functional theory.J Phys Chem A. 2011 Sep 1;115(34):9467-78. doi: 10.1021/jp111878h. Epub 2011 Mar 29. J Phys Chem A. 2011. PMID: 21446683
-
Two-dimensional infrared spectroscopy of intermolecular hydrogen bonds in the condensed phase.Acc Chem Res. 2009 Sep 15;42(9):1220-8. doi: 10.1021/ar900006u. Acc Chem Res. 2009. PMID: 19425543 Review.
-
UV-excitation from an experimental perspective: frequency resolved.Top Curr Chem. 2015;355:33-56. doi: 10.1007/128_2014_560. Top Curr Chem. 2015. PMID: 25388412 Review.
References
-
- Joyce G. F., Nature 1989, 338, 217–224. - PubMed
-
- Walter M. R., in Earth's Earliest Biosphere (Eds:Schopf J. W.,), Princeton University Press, 1983, p. 197.
-
- Buick R., Dunlop J. S. R., Groves D. I., Alcheringa. 1981, 5, 161–181.<
-
- Schidlowski M., Appel P. W. U., Eichmann R., Junge C. E., Geochim. Cosmochim. Acta 1979, 43, 189–199.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

