A First-in-Man Clinical Evaluation of Sirolimus and Ascorbic Acid-Eluting Stent Systems: a Multicenter, Subject-Blinded, Randomized Study
- PMID: 34854580
- PMCID: PMC8636762
- DOI: 10.4070/kcj.2021.0161
A First-in-Man Clinical Evaluation of Sirolimus and Ascorbic Acid-Eluting Stent Systems: a Multicenter, Subject-Blinded, Randomized Study
Abstract
Background and objectives: This clinical trial was conducted to evaluate the safety and efficacy of D+Storm™ drug-eluting stent (DES) and BioMatrix Flex™ DES.
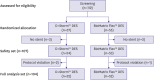
Methods: This study was a multicenter, subject-single-blind, randomized, and confirmed comparative clinical trial. According to the inclusion criteria, those diagnosed with stable angina, unstable angina, silent ischemia, or non-ST-segment myocardial infarction were selected among patients with coronary artery stenosis as subjects. Among the subjects with 50% stenosis on coronary angiography, the experiment was performed on those who had a lesion with reference vessel 2.5-4.0 mm in diameter and ≤40 mm in length. The primary endpoint was an in-segment late loss and the secondary endpoints were in-stent late lumen loss, stent malapposition, the incidence of mortality, myocardial infarction, reoperation, and stent thrombosis at 36 weeks.
Results: 57 patients in the D+Storm™ DES group and 55 patients in the BioMatrix Flex™ DES group were enrolled in the study. Fifty-seven patients in the D+Storm™ DES group and Fifty-five patients in the BioMatrix Flex™ DES group were enrolled in the study. An average of in-segment late lumen loss was 0.08±0.13 mm in the D+Storm™ DES group and 0.14±0.32 mm in the BioMatrix Flex™ DES group with no significant difference between the 2 groups (p=0.879). In addition, there was no significant difference in adverse events between D+Storm™ DES and BioMatrix Flex™ DES.
Conclusions: This study demonstrated the clinical effectiveness and safety of D+Storm™ DES implantation in patients with coronary artery disease over a 36-week follow-up period.
Keywords: Absorbable implants; Ascorbic acid; Coronary artery disease; Drug-eluting stents; Sirolimus.
Copyright © 2021. The Korean Society of Cardiology.
Conflict of interest statement
Jun-kyu Park and Ji Hyun Youn are researchers at CG Bio, a manufacturer of D+Storm DES. This clinical trial was funded by CG Bio. The other authors report no conflicts.
Figures
References
-
- Shin ES. Current status of coronary stent. Korean J Med. 2015;89:282–290.
-
- Choi C, Nah JW, Park JK. Development trends of the stent for coronary artery. Korean Ind Chem News. 2015;18:10–24.
-
- Moses JW, Leon MB, Popma JJ, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. - PubMed
-
- Bedair TM, Kang SN, Joung YK, Han DK. A promising approach for improving the coating stability and in vivo performance of biodegradable polymer-coated sirolimus-eluting stent. J Biomed Nanotechnol. 2016;12:2015–2028. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources