Liver-targeted delivery of asiatic acid nanostructured lipid carrier for the treatment of liver fibrosis
- PMID: 34854788
- PMCID: PMC8648005
- DOI: 10.1080/10717544.2021.2008054
Liver-targeted delivery of asiatic acid nanostructured lipid carrier for the treatment of liver fibrosis
Abstract
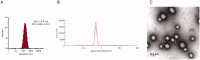
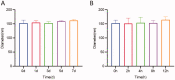
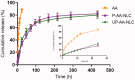
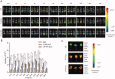
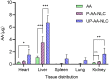
Liver fibrosis is a major global health concern. Management of chronic liver disease is severely restricted in clinics due to ineffective treatment approaches. However, a lack of targeted therapy may aggravate this condition. Asiatic acid (AA), a pentacyclic triterpenoid acid, can effectively protect the liver from hepatic disorders. However, the pharmaceutical application of AA is limited by low oral bioavailability and poor targeting efficiency. This study synthesized a novel liver-targeting material from PEG-SA, chemically linked to ursodeoxycholic acid (UA), and utilized it to modify AA nanostructured lipid carriers (UP-AA-NLC) with enhanced targeting and improved efficacy. The formulation of UP-AA-NLC was optimized via the Box-Behnken Experimental Design (BBD) and characterized by size, zeta potential, TEM, DSC, and XRD. Furthermore, in vitro antifibrotic activity and proliferation of AA and NLCs were assessed in LX-2 cells. The addition of UP-AA-NLC significantly stimulated the TGF-beta1-induced expression of α-SMA, FN1, and Col I α1. In vivo near-infrared fluorescence imaging and distribution trials in rats demonstrated that UP-AA-NLC could significantly improve oral absorption and liver-targeting efficiency. Oral UP-AA-NLC greatly alleviated carbon tetrachloride-induced liver injury and fibrosis in rats in a dosage-dependent manner, as reflected by serum biochemical parameters (AST, ALT, and ALB), histopathological features (H&E and Masson staining), and antioxidant activity parameters (SOD and MDA). Also, treatment with UP-AA-NLC lowered liver hydroxyproline levels, demonstrating a reduction of collagen accumulation in the fibrotic liver. Collectively, optimized UP-AA-NLC has potential application prospects in liver-targeted therapy and holds great promise as a drug delivery system for treating liver diseases.
Keywords: Box–Behnken design; Liver-targeted therapy; asiatic acid; liver fibrosis; nanostructured lipid carrier.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures








References
-
- Bedossa P, Poynard T. (1996). An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology 24:289–93. - PubMed
-
- Chasseaud LF, Fry BJ, Hawkins DR, et al. (1971). The metabolism of asiatic acid, madecassic acid and asiaticoside in the rat. Drug Res 21:1379–84. - PubMed
-
- Chen XX, Zhang YW, Zhao PF, et al. (2020). Preparation and evaluation of PEGylated asiatic acid nanostructured lipid carriers on anti-fibrosis effects. Drug Dev Ind Pharm 46:57–69. - PubMed
-
- Chen Y, Lu Y, Chen J, et al. (2009). Enhanced bioavailability of the poorly water-soluble drug fenofibrate by using liposomes containing a bile salt. Int J Pharm 376:153–60. - PubMed
-
- Chen ZP, Zhu JB, Chen HX, et al. (2010). Synthesis of a novel polymer bile salts-(polyethylene glycol)2000-bile salts and its application to the liver-selective targeting of liposomal DDB. Drug Dev Ind Pharm 36:657–65. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
