A Mobile App for Wound and Symptom Surveillance After Colorectal Surgery: Protocol for a Feasibility Randomized Controlled Trial
- PMID: 34854816
- PMCID: PMC8763310
- DOI: 10.2196/26717
A Mobile App for Wound and Symptom Surveillance After Colorectal Surgery: Protocol for a Feasibility Randomized Controlled Trial
Abstract
Background: Surgical site infections (SSIs) are the most common nosocomial infection and occur in 16.3% of patients undergoing colorectal surgery at our institution (The Ottawa Hospital), the majority of which are identified after discharge from hospital. Patients who suspect having an SSI generally present to the emergency department or surgery clinic. Both options for in-person interaction are costly to the health care system and patients. A mobile app, how2trak, has proven to be beneficial for patients with complex wounds at our institution by facilitating at-home monitoring and virtual consultations.
Objective: This study aims to assess the feasibility of a randomized controlled trial to assess if how2trak can improve patients' experience and increase detection of SSIs after colorectal surgery while reducing patients' risk of COVID-19 exposure.
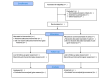
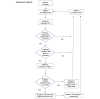
Methods: In this single-center prospective feasibility trial, eligible patients undergoing colorectal surgery will be randomized to either standard care or how2trak postoperative monitoring of their incision, symptoms, and ostomy function. Patient self-assessments will be monitored by a nurse specialized in wound and ostomy care who will follow-up with patients with a suspected SSI. The primary outcome is feasibility as measured by enrollment, randomization, app usability, data extraction, and resource capacity.
Results: This study was approved by our institution's ethics board on February 26, 2021, and received support from The Ottawa Hospital Innovation and Care Funding on November 12, 2021. Recruitment started June 3, 2021, and 29 were patients enrolled as of September 2021. We expect to publish results in spring 2022.
Conclusions: This study will determine the feasibility of using a mobile app to monitor patients' wounds and detect SSIs after colorectal surgery. If feasible, we plan to assess if this mobile app facilitates SSI detection, enhances patient experience, and optimizes their care.
Trial registration: ClinicalTrials.gov NCT04869774; https://clinicaltrials.gov/ct2/show/NCT04869774.
International registered report identifier (irrid): DERR1-10.2196/26717.
Keywords: COVID-19; app; colorectal surgery; eHealth; feasibility; infection; mobile app; patient experience; randomized controlled trial; surgery; surgical site infection; surveillance; tracking; transmission; wound.
©Heather Anne Valk, Carlos Garcia-Ochoa, Jessica Fontaine Calder, Toba Miller, Babak Rashidi, Corrine McIsaac, Reilly Musselman. Originally published in JMIR Research Protocols (https://www.researchprotocols.org), 14.01.2022.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures
Similar articles
-
The effect of mobile app home monitoring on number of in-person visits following ambulatory surgery: protocol for a randomized controlled trial.JMIR Res Protoc. 2015 Jun 3;4(2):e65. doi: 10.2196/resprot.4352. JMIR Res Protoc. 2015. PMID: 26040252 Free PMC article.
-
App-Controlled Treatment Monitoring and Support for Head and Neck Cancer Patients (APCOT): Protocol for a Prospective Randomized Controlled Trial.JMIR Res Protoc. 2020 Dec 9;9(12):e21693. doi: 10.2196/21693. JMIR Res Protoc. 2020. PMID: 33295291 Free PMC article.
-
Post-Caesarean Section Surgical Site Infection Surveillance Using an Online Database and Mobile Phone Technology.J Obstet Gynaecol Can. 2017 Aug;39(8):645-651.e1. doi: 10.1016/j.jogc.2016.12.037. J Obstet Gynaecol Can. 2017. PMID: 28729097
-
An Individual Cognitive Stimulation Therapy App for People With Dementia and Their Carers: Protocol for a Feasibility Randomized Controlled Trial.JMIR Res Protoc. 2021 Apr 8;10(4):e24628. doi: 10.2196/24628. JMIR Res Protoc. 2021. PMID: 33830058 Free PMC article.
-
Feasibility of app-based home monitoring after abdominal surgery: A systematic review.Am J Surg. 2024 Nov;237:115764. doi: 10.1016/j.amjsurg.2024.05.005. Epub 2024 May 16. Am J Surg. 2024. PMID: 38830790
Cited by
-
Mobile applications in gastrointestinal surgery: a systematic review.Surg Endosc. 2023 Jun;37(6):4224-4248. doi: 10.1007/s00464-023-10007-y. Epub 2023 Apr 4. Surg Endosc. 2023. PMID: 37016081 Free PMC article.
References
-
- Petrosyan Y, Thavorn K, Maclure M, Smith G, McIsaac D, Schramm D, Moloo H, Preston R, Forster A. Long-term Health Outcomes and Health System Costs Associated With Surgical Site Infections: A Retrospective Cohort Study. Ann Surg. 2021 May 01;273(5):917–923. doi: 10.1097/SLA.0000000000003285.00000658-202105000-00014 - DOI - PubMed
-
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014 Mar 27;370(13):1198–208. doi: 10.1056/NEJMoa1306801. http://europepmc.org/abstract/MED/24670166 - DOI - PMC - PubMed
-
- Gravel D, Taylor G, Ofner M, Johnston L, Loeb M, Roth VR, Stegenga J, Bryce E, Canadian Nosocomial Infection Surveillance Program. Matlow A. Point prevalence survey for healthcare-associated infections within Canadian adult acute-care hospitals. J Hosp Infect. 2007 Jul;66(3):243–8. doi: 10.1016/j.jhin.2007.04.008.S0195-6701(07)00126-0 - DOI - PubMed
-
- Annual Epidemiological Report on Communicable Diseases in Europe. Stockholm: European Centre for Disease Prevention and Control; 2016. [2021-12-29]. Healthcare-associated infections: surgical site infections - Annual Epidemiological Report for 2016. https://www.ecdc.europa.eu/en/publications-data/healthcare-associated-in... .
Associated data
LinkOut - more resources
Full Text Sources
Medical

