EphA2 and Ephrin-A5 Guide Eye Lens Suture Alignment and Influence Whole Lens Resilience
- PMID: 34854885
- PMCID: PMC8648058
- DOI: 10.1167/iovs.62.15.3
EphA2 and Ephrin-A5 Guide Eye Lens Suture Alignment and Influence Whole Lens Resilience
Abstract
Purpose: Fine focusing of light by the eye lens onto the retina relies on the ability of the lens to change shape during the process of accommodation. Little is known about the cellular structures that regulate elasticity and resilience. We tested whether Eph-ephrin signaling is involved in lens biomechanical properties.
Methods: We used confocal microscopy and tissue mechanical testing to examine mouse lenses with genetic disruption of EphA2 or ephrin-A5.
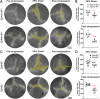
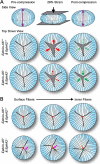
Results: Confocal imaging revealed misalignment of the suture between each shell of newly added fiber cells in knockout lenses. Despite having disordered sutures, loss of EphA2 or ephrin-A5 did not affect lens stiffness. Surprisingly, knockout lenses were more resilient and recovered almost completely after load removal. Confocal microscopy and quantitative image analysis from live lenses before, during, and after compression revealed that knockout lenses had misaligned Y-sutures, leading to a change in force distribution during compression. Knockout lenses displayed decreased separation of fiber cell tips at the anterior suture at high loads and had more complete recovery after load removal, which leads to improved whole-lens resiliency.
Conclusions: EphA2 and ephrin-A5 are needed for normal patterning of fiber cell tips and the formation of a well-aligned Y-suture with fiber tips stacked on top of previous generations of fiber cells. The misalignment of lens sutures leads to increased resilience after compression. The data suggest that alignment of the Y-suture may constrain the overall elasticity and resilience of the lens.
Conflict of interest statement
Disclosure:
Figures



References
-
- Heys KR, Cram SL, Truscott RJ.. Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol Vis. 2004; 10: 956–963. - PubMed
-
- Glasser A, Campbell MC.. Biometric, optical and physical changes in the isolated human crystalline lens with age in relation to presbyopia. Vision Res. 1999; 39: 1991–2015. - PubMed
-
- Weeber HA, Eckert G, Soergel F, Meyer CH, Pechhold W, van der Heijde RG.. Dynamic mechanical properties of human lenses. Exp Eye Res. 2005; 80: 425–434. - PubMed
-
- Heys KR, Friedrich MG, Truscott RJ.. Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat shock protein, alpha-crystallin, in maintaining lens flexibility. Aging Cell. 2007; 6: 807–815. - PubMed
-
- Pierscionek BK. Age-related response of human lenses to stretching forces. Exp Eye Res. 1995; 60: 325–332. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

