Association Between Sex and Immune Checkpoint Inhibitor Outcomes for Patients With Melanoma
- PMID: 34854905
- PMCID: PMC8640892
- DOI: 10.1001/jamanetworkopen.2021.36823
Association Between Sex and Immune Checkpoint Inhibitor Outcomes for Patients With Melanoma
Abstract
Importance: Immune checkpoint inhibitors (ICIs) have revolutionized melanoma treatment and are now standard of care. Although sex is associated with immune function and immune-related diseases, the interaction between sex and ICIs is understudied.
Objective: To examine whether cancer immunotherapy effectiveness varies between female and male patients with advanced melanoma treated with either nivolumab plus ipilimumab combination therapy or anti-programmed cell death protein 1 (PD-1) therapy (namely, pembrolizumab or nivolumab).
Design, setting, and participants: The study population consisted of 1369 older adults (aged ≥65 years) with a record of melanoma diagnosis from January 1, 1991, to December 31, 2015, in the Surveillance, Epidemiology, and End Results-Medicare linked database. Patients with a diagnosis of stage III or stage IV melanoma and a claims record showing nivolumab plus ipilimumab combination therapy or anti-PD-1 therapy (ie, pembrolizumab or nivolumab) as their last type of ICI prescribed were included in the analyses. Patients were followed up through December 31, 2017, for the overall survival analysis. Statistical analysis was performed from September 19, 2019, to February 20, 2021.
Exposures: Sex, last prescribed ICI, and prior use of ipilimumab.
Main outcomes and measures: The primary outcome was overall survival, defined as time from the index date until death from any cause, with patients censored at the end of the study (December 31, 2017). Cox proportional hazards regression modeling was used to examine the association of sex with ICI outcomes while adjusting for prior use of ipilimumab, age at ICI initiation, Charlson Comorbidity Index, cancer stage at the time of diagnosis, and autoimmune disease diagnosis.
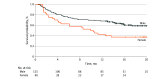
Results: Among the 1369 patients in the study (982 men [71.7%]; median age, 75 years [IQR, 69-82 years]), the outcome of nivolumab plus ipilimumab combination therapy depended on sex (Wald χ2 = 9.48; P = .009 for interaction). The mortality hazard ratio (HR) for women with prior ipilimumab use receiving combination therapy was 2.06 times (95% CI, 1.28-3.32; P = .003) higher than their male counterparts. No significant difference was observed between women and men receiving anti-PD-1 therapy with (HR, 0.97 [95% CI, 0.68-1.38]; P = .85) or without prior ipilimumab use (HR, 0.85 [95% CI, 0.67-1.07]; P = .16). For women with prior ipilimumab use, combination therapy was associated with 2.82 times higher mortality hazards than anti-PD-1 therapy (95% CI, 1.73-4.60). No statistically significant difference was seen in mortality risk between anti-PD-1 therapy and combination therapy for men.
Conclusions and relevance: This cohort study suggests that female patients with advanced melanoma may not benefit as much from combination ICIs as male patients would. Tumor mutation burden or estrogen level may serve as an important biomarker associated with ICI response in metastatic melanoma.
Conflict of interest statement
Figures

Similar articles
-
Effectiveness of Immune Checkpoint Inhibitor with Anti-PD-1 Monotherapy or in Combination with Ipilimumab in Younger versus Older Adults with Advanced Melanoma.Curr Oncol. 2023 Sep 30;30(10):8936-8947. doi: 10.3390/curroncol30100646. Curr Oncol. 2023. PMID: 37887546 Free PMC article.
-
Real-world outcomes in patients with melanoma brain metastasis: a US multisite retrospective chart review study of systemic treatments.BMJ Open. 2025 Jan 30;15(1):e091098. doi: 10.1136/bmjopen-2024-091098. BMJ Open. 2025. PMID: 39890146 Free PMC article.
-
Treatment-Free Survival After Nivolumab vs Pembrolizumab vs Nivolumab-Ipilimumab for Advanced Melanoma.JAMA Netw Open. 2023 Jun 1;6(6):e2319607. doi: 10.1001/jamanetworkopen.2023.19607. JAMA Netw Open. 2023. PMID: 37351883 Free PMC article.
-
Systematic review and meta-analysis efficacy and safety of immune checkpoint inhibitors in advanced melanoma patients with anti-PD-1 progression: a systematic review and meta-analysis.Clin Transl Oncol. 2021 Sep;23(9):1885-1904. doi: 10.1007/s12094-021-02598-6. Epub 2021 Apr 20. Clin Transl Oncol. 2021. PMID: 33877531
-
Immune Checkpoint Inhibitors and Immune-Related Adverse Events in Patients With Advanced Melanoma: A Systematic Review and Network Meta-analysis.JAMA Netw Open. 2020 Mar 2;3(3):e201611. doi: 10.1001/jamanetworkopen.2020.1611. JAMA Netw Open. 2020. PMID: 32211869 Free PMC article.
Cited by
-
Fixed-Dose Versus Weight-Adapted Immune Checkpoint Inhibitor Therapy in Melanoma: A Retrospective Monocentric Analysis of Efficacy and Immune-Related Adverse Events.Cancers (Basel). 2025 Mar 28;17(7):1147. doi: 10.3390/cancers17071147. Cancers (Basel). 2025. PMID: 40227712 Free PMC article.
-
Gradient differences of immunotherapy efficacy in metastatic melanoma related to sunlight exposure pattern: A population-based study.Front Oncol. 2023 Jan 5;12:1086664. doi: 10.3389/fonc.2022.1086664. eCollection 2022. Front Oncol. 2023. PMID: 36686834 Free PMC article.
-
Gender oncology: recommendations and consensus of the Italian Association of Medical Oncology (AIOM).ESMO Open. 2024 Feb;9(2):102243. doi: 10.1016/j.esmoop.2024.102243. Epub 2024 Feb 23. ESMO Open. 2024. PMID: 38394984 Free PMC article.
-
Immune checkpoint inhibitors in metastatic melanoma therapy (Review).Med Int (Lond). 2024 Feb 9;4(2):13. doi: 10.3892/mi.2024.137. eCollection 2024 Mar-Apr. Med Int (Lond). 2024. PMID: 38410760 Free PMC article. Review.
-
The impact of sex on HIV immunopathogenesis and therapeutic interventions.J Clin Invest. 2024 Sep 17;134(18):e180075. doi: 10.1172/JCI180075. J Clin Invest. 2024. PMID: 39286972 Free PMC article. Review.
References
-
- Warner AB, Postow MA. Combination controversies: checkpoint inhibition alone or in combination for the treatment of melanoma? Oncology (Williston Park). 2018;32(5):228-234. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

