Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study
- PMID: 34854921
- PMCID: PMC8640949
- DOI: 10.1001/jamaoncol.2021.5771
Association of COVID-19 Vaccination With SARS-CoV-2 Infection in Patients With Cancer: A US Nationwide Veterans Affairs Study
Abstract
Importance: Patients with cancer are at increased risk for severe COVID-19, but it is unknown whether SARS-CoV-2 vaccination is effective for them.
Objective: To determine the association between SARS-CoV-2 vaccination and SARS-CoV-2 infections among a population of Veterans Affairs (VA) patients with cancer.
Design, setting, and participants: Retrospective, multicenter, nationwide cohort study of SARS-CoV-2 vaccination and infection among patients in the VA health care system from December 15, 2020, to May 4, 2021. All adults with solid tumors or hematologic cancer who received systemic cancer-directed therapy from August 15, 2010, to May 4, 2021, and were alive and without a documented SARS-CoV-2 positive result as of December 15, 2020, were eligible for inclusion. Each day between December 15, 2020, and May 4, 2021, newly vaccinated patients were matched 1:1 with unvaccinated or not yet vaccinated controls based on age, race and ethnicity, VA facility, rurality of home address, cancer type, and treatment type/timing.
Exposures: Receipt of a SARS-CoV-2 vaccine.
Main outcomes and measures: The primary outcome was documented SARS-CoV-2 infection. A proxy for vaccine effectiveness was defined as 1 minus the risk ratio of SARS-CoV-2 infection for vaccinated individuals compared with unvaccinated controls.
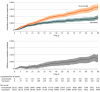
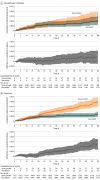
Results: A total of 184 485 patients met eligibility criteria, and 113 796 were vaccinated. Of these, 29 152 vaccinated patients (median [IQR] age, 74.1 [70.2-79.3] years; 95% were men; 71% were non-Hispanic White individuals) were matched 1:1 to unvaccinated or not yet vaccinated controls. As of a median 47 days of follow-up, 436 SARS-CoV-2 infections were detected in the matched cohort (161 infections in vaccinated patients vs 275 in unvaccinated patients). There were 17 COVID-19-related deaths in the vaccinated group vs 27 COVID-19-related deaths in the unvaccinated group. Overall vaccine effectiveness in the matched cohort was 58% (95% CI, 39% to 72%) starting 14 days after the second dose. Patients who received chemotherapy within 3 months prior to the first vaccination dose were estimated to have a vaccine effectiveness of 57% (95% CI, -23% to 90%) starting 14 days after the second dose vs 76% (95% CI, 50% to 91%) for those receiving endocrine therapy and 85% (95% CI, 29% to 100%) for those who had not received systemic therapy for at least 6 months prior.
Conclusions and relevance: In this cohort study, COVID-19 vaccination was associated with lower SARS-CoV-2 infection rates in patients with cancer. Some immunosuppressed subgroups may remain at early risk for COVID-19 despite vaccination, and consideration should be given to additional risk reduction strategies, such as serologic testing for vaccine response and a third vaccine dose to optimize outcomes.
Conflict of interest statement
Figures
Similar articles
-
Coverage and Estimated Effectiveness of mRNA COVID-19 Vaccines Among US Veterans.JAMA Netw Open. 2021 Oct 1;4(10):e2128391. doi: 10.1001/jamanetworkopen.2021.28391. JAMA Netw Open. 2021. PMID: 34613401 Free PMC article.
-
Factors Associated With Severe COVID-19 Among Vaccinated Adults Treated in US Veterans Affairs Hospitals.JAMA Netw Open. 2022 Oct 3;5(10):e2240037. doi: 10.1001/jamanetworkopen.2022.40037. JAMA Netw Open. 2022. PMID: 36264571 Free PMC article.
-
COVID-19 Vaccination Effectiveness Against Infection or Death in a National U.S. Health Care System : A Target Trial Emulation Study.Ann Intern Med. 2022 Mar;175(3):352-361. doi: 10.7326/M21-3256. Epub 2021 Dec 21. Ann Intern Med. 2022. PMID: 34928700 Free PMC article.
-
SARS-CoV-2 primary and breakthrough infections in patients with cancer: Implications for patient care.Best Pract Res Clin Haematol. 2022 Sep;35(3):101384. doi: 10.1016/j.beha.2022.101384. Epub 2022 Sep 29. Best Pract Res Clin Haematol. 2022. PMID: 36494154 Free PMC article. Review.
-
Risk of myocarditis and pericarditis in mRNA COVID-19-vaccinated and unvaccinated populations: a systematic review and meta-analysis.BMJ Open. 2023 Jun 20;13(6):e065687. doi: 10.1136/bmjopen-2022-065687. BMJ Open. 2023. PMID: 37339840 Free PMC article.
Cited by
-
Recent common human coronavirus infection protects against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: A Veterans Affairs cohort study.Proc Natl Acad Sci U S A. 2022 Nov 16;119(46):e2213783119. doi: 10.1073/pnas.2213783119. Epub 2022 Nov 7. Proc Natl Acad Sci U S A. 2022. PMID: 36343242 Free PMC article. No abstract available.
-
The Interplay of Lung Cancer, COVID-19, and Vaccines.Int J Mol Sci. 2022 Dec 1;23(23):15067. doi: 10.3390/ijms232315067. Int J Mol Sci. 2022. PMID: 36499394 Free PMC article. Review.
-
Associations of COVID-19 vaccination with risks for post-infectious cardiovascular complications: an international cohort study in cancer patients with SARS-CoV-2 infection.Lancet Reg Health Am. 2025 Mar 6;44:101038. doi: 10.1016/j.lana.2025.101038. eCollection 2025 Apr. Lancet Reg Health Am. 2025. PMID: 40124588 Free PMC article.
-
Immunogenicity after a Third COVID-19 mRNA Booster in Solid Cancer Patients Who Previously Received the Primary Heterologous CoronaVac/ChAdOx1 Vaccine.Vaccines (Basel). 2022 Sep 26;10(10):1613. doi: 10.3390/vaccines10101613. Vaccines (Basel). 2022. PMID: 36298478 Free PMC article.
-
Updated International Society of Geriatric Oncology COVID-19 working group recommendations on COVID-19 vaccination among older adults with cancer.J Geriatr Oncol. 2022 Sep;13(7):1054-1057. doi: 10.1016/j.jgo.2022.07.005. Epub 2022 Jul 15. J Geriatr Oncol. 2022. PMID: 35853816 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

